3) What is a reaction rate? a) how fast reactants are used up, or products are formed b) orders of the reaction c) mechanism of the reaction d) whether the reaction is reactants or products favored 4) How do we know concentrations have changed in the iodine clock experiment? a) concentrations are measured after a specified time b) a dramatic and instant color change shows that that reactants are used up and product forme c) by performing calculations d) by reading a graph 5) What is a reaction mechanism? a) how fast reactants are used up, or products are formed b) orders of the reaction c) series of steps by which reactant atoms break existing bonds and form new bonds d) whether the reaction is reactants or products favored
3) What is a reaction rate? a) how fast reactants are used up, or products are formed b) orders of the reaction c) mechanism of the reaction d) whether the reaction is reactants or products favored 4) How do we know concentrations have changed in the iodine clock experiment? a) concentrations are measured after a specified time b) a dramatic and instant color change shows that that reactants are used up and product forme c) by performing calculations d) by reading a graph 5) What is a reaction mechanism? a) how fast reactants are used up, or products are formed b) orders of the reaction c) series of steps by which reactant atoms break existing bonds and form new bonds d) whether the reaction is reactants or products favored
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter13: Chemical Kinetics
Section: Chapter Questions
Problem 13.98QE
Related questions
Question
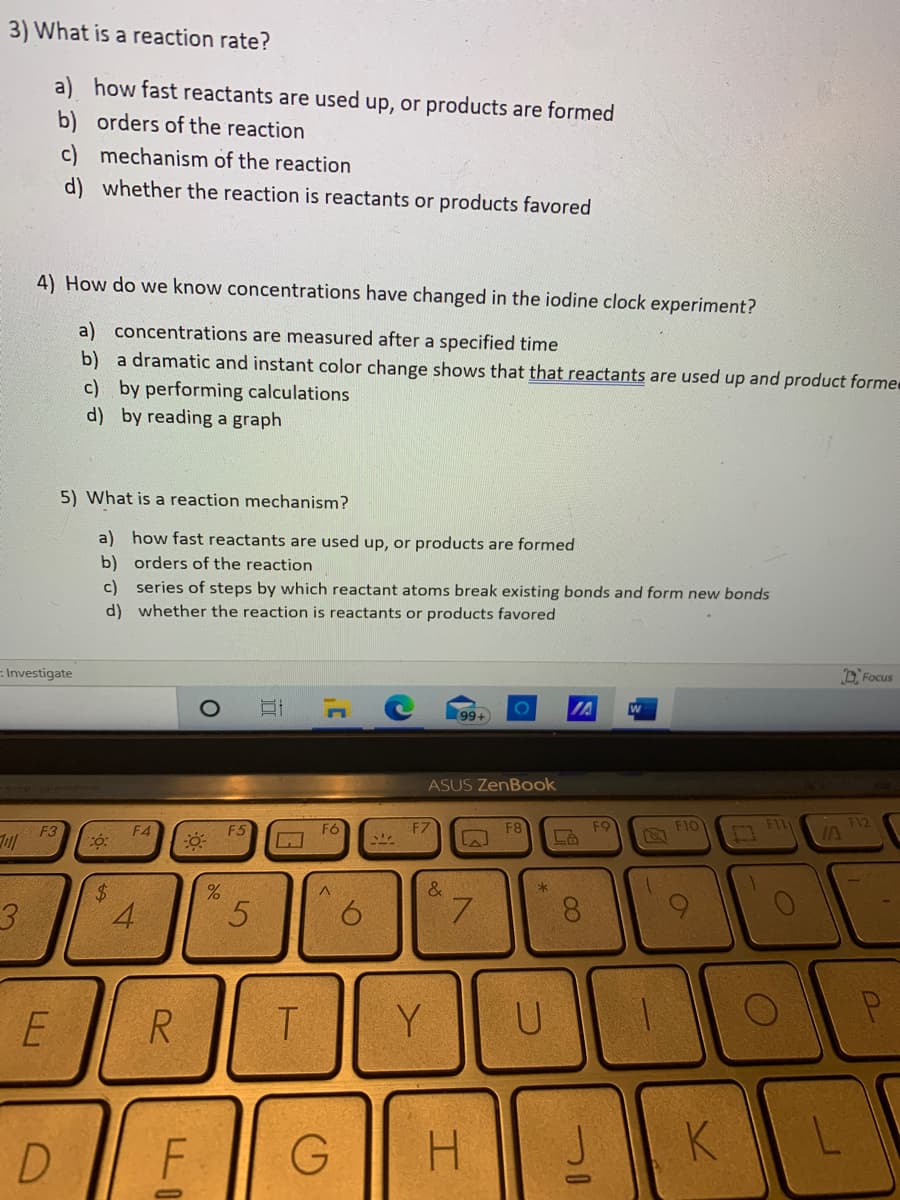
Transcribed Image Text:3) What is a reaction rate?
a) how fast reactants are used up, or products are formed
b) orders of the reaction
c) mechanism of the reaction
d) whether the reaction is reactants or products favored
4) How do we know concentrations have changed in the iodine clock experiment?
a) concentrations are measured after a specified time
b) a dramatic and instant color change shows that that reactants are used up and product forme
c) by performing calculations
d) by reading a graph
5) What is a reaction mechanism?
a) how fast reactants are used up, or products are formed
b) orders of the reaction
c) series of steps by which reactant atoms break existing bonds and form new bonds
d) whether the reaction is reactants or products favored
Investigate
D Focus
IA
99+
ASUS ZenBook
F4
F5
F6
F7
F8
F9
FIO
F12
F3
%$4
3.
4.
7.
8.
R.
Y
D.
F
H.
JK
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co