3. Compute for the amount of increase in concentration of CO2 in ppm and in metric tons. Weight of CO2 (in billion metric tons) 747.63 Year Atmospheric CO2 concentration ppm ppm 1966 351 2014 450 958.50 amount of increase from 1966 to 2014
3. Compute for the amount of increase in concentration of CO2 in ppm and in metric tons. Weight of CO2 (in billion metric tons) 747.63 Year Atmospheric CO2 concentration ppm ppm 1966 351 2014 450 958.50 amount of increase from 1966 to 2014
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 129E
Related questions
Question
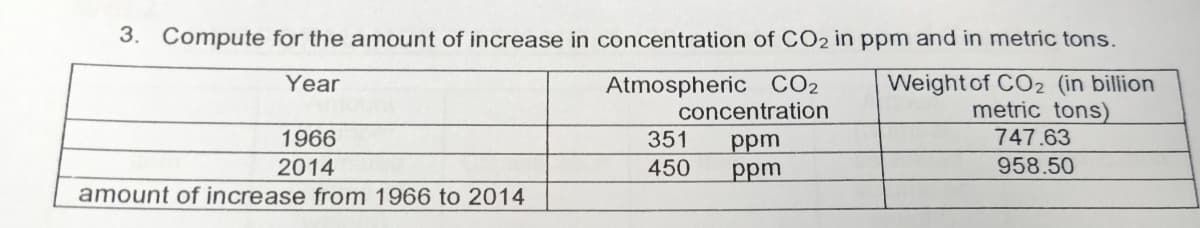
Transcribed Image Text:3. Compute for the amount of increase in concentration of CO2 in ppm and in metric tons.
Weight of CO2 (in bilion
metric tons)
Atmospheric CO2
concentration
ppm
ppm
Year
1966
351
747.63
2014
450
958.50
amount of increase from 1966 to 2014
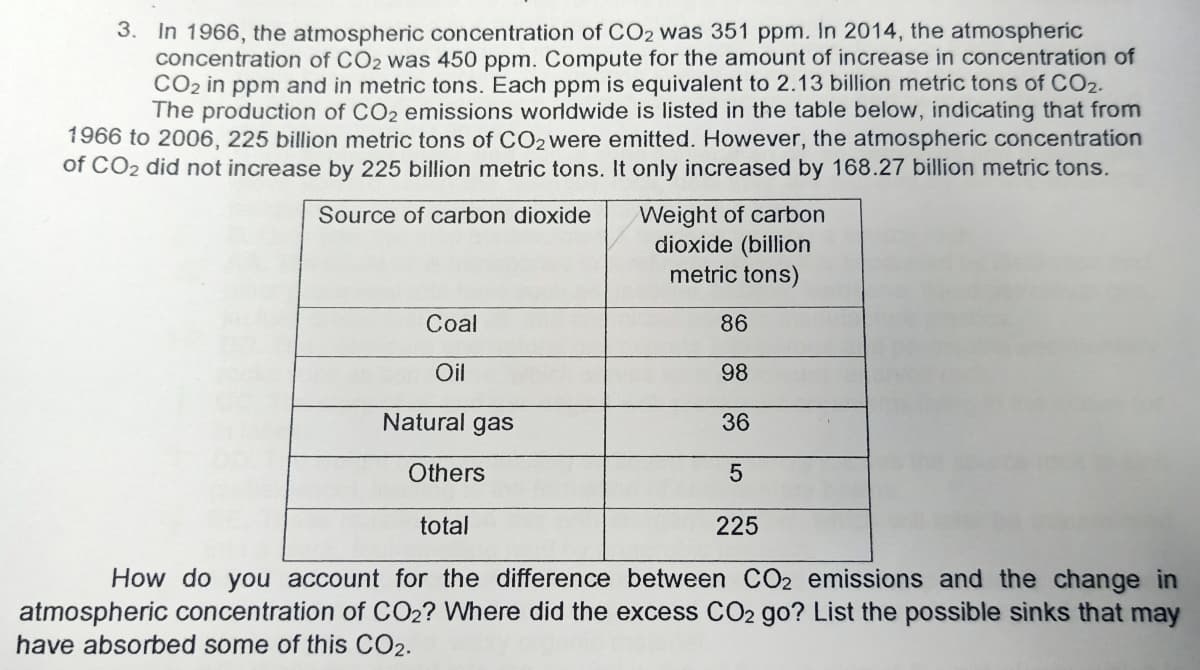
Transcribed Image Text:3. In 1966, the atmospheric concentration of CO2 was 351 ppm. In 2014, the atmospheric
concentration of CO2 was 450 ppm. Compute for the amount of increase in concentration of
CO2 in ppm and in metric tons. Each ppm is equivalent to 2.13 billion metric tons of CO2.
The production of CO2 emissions worldwide is listed in the table below, indicating that from
1966 to 2006, 225 billion metric tons of CO2were emitted. However, the atmospheric concentration
of CO2 did not increase by 225 billion metric tons. It only increased by 168.27 billion metric tons.
Weight of carbon
dioxide (billion
metric tons)
Source of carbon dioxide
Coal
86
Oil
98
Natural gas
36
Others
total
225
How do you account for the difference between CO2 emissions and the change in
atmospheric concentration of CO2? Where did the excess CO2 go? List the possible sinks that may
have absorbed some of this CO2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
