3. Consider the a bonding of the cis-1,3-butadiene molecule of C2v symmetry in the xz plane with the carbon atoms numbered as indicated CH2 4 A.. determine the energies of the x orbitals of this molecule. Write this in terms of a and B. Using the Hückel method write the secular determinant that must be solved to B. Often a is set to zero and B is set to unity. In this case what would an energy value of -1.62 correspond to relative to other energy values? C. The energy solutions for part A are 1.62B, 0.62B, -0.62B, and -1.62B. Draw an energy diagram for these levels and show the location of the electrons. What is the a bond stabilization for this molecule in B units? D. linear combinations of atomic orbitals. For each indicate which energy level it corresponds to and label it as bonding, antibonding, or non-bonding. The symmetry species for the orbitals are 2A2+ 2B2. Label each orbital with the correct symmetry species. The wave-functions corresponding to the energy levels are shown below as
3. Consider the a bonding of the cis-1,3-butadiene molecule of C2v symmetry in the xz plane with the carbon atoms numbered as indicated CH2 4 A.. determine the energies of the x orbitals of this molecule. Write this in terms of a and B. Using the Hückel method write the secular determinant that must be solved to B. Often a is set to zero and B is set to unity. In this case what would an energy value of -1.62 correspond to relative to other energy values? C. The energy solutions for part A are 1.62B, 0.62B, -0.62B, and -1.62B. Draw an energy diagram for these levels and show the location of the electrons. What is the a bond stabilization for this molecule in B units? D. linear combinations of atomic orbitals. For each indicate which energy level it corresponds to and label it as bonding, antibonding, or non-bonding. The symmetry species for the orbitals are 2A2+ 2B2. Label each orbital with the correct symmetry species. The wave-functions corresponding to the energy levels are shown below as
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter15: Introduction To Electronic Spectroscopy And Structure
Section: Chapter Questions
Problem 15.2E: Linearly polarized light can be assigned a specific irreducible representation of a symmetry point...
Related questions
Question
please solve part 4
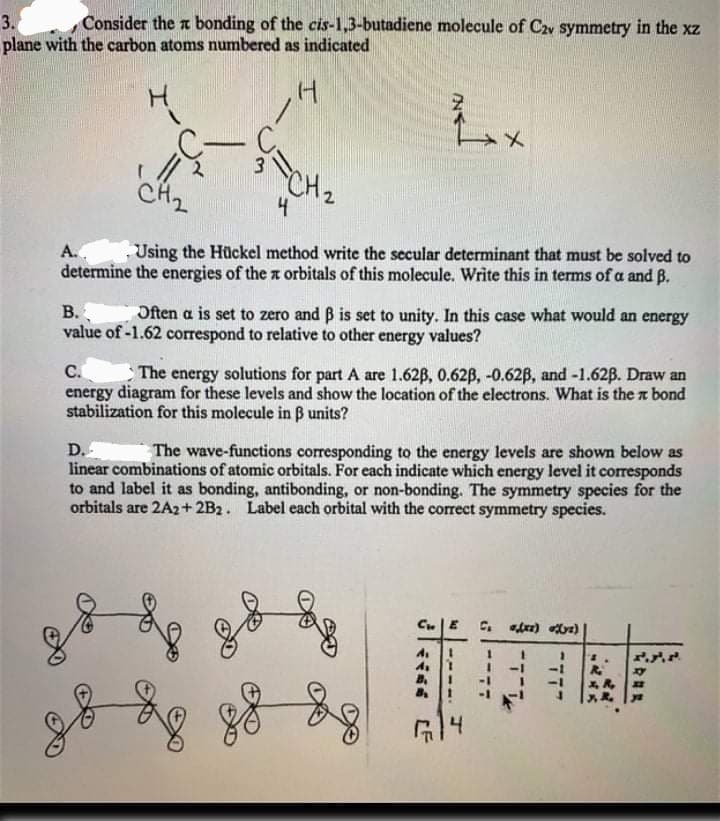
Transcribed Image Text:3.
Consider the a bonding of the cis-1,3-butadiene molecule of C2v symmetry in the xz
plane with the carbon atoms numbered as indicated
CH2
A..
Using the Hückel method write the secular determinant that must be solved to
determine the energies of the x orbitals of this molecule. Write this in terms of a and B.
B.
Often a is set to zero and B is set to unity. In this case what would an energy
value of -1.62 correspond to relative to other energy values?
C.
The energy solutions for part A are 1.62B, 0.62B, -0.62B, and -1.62B. Draw an
energy diagram for these levels and show the location of the electrons. What is the a bond
stabilization for this molecule in B units?
D.
linear combinations of atomic orbitals. For each indicate which energy level it corresponds
to and label it as bonding, antibonding, or non-bonding. The symmetry species for the
orbitals are 2A2+ 2B2. Label each orbital with the correct symmetry species.
The wave-functions corresponding to the energy levels are shown below as
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning