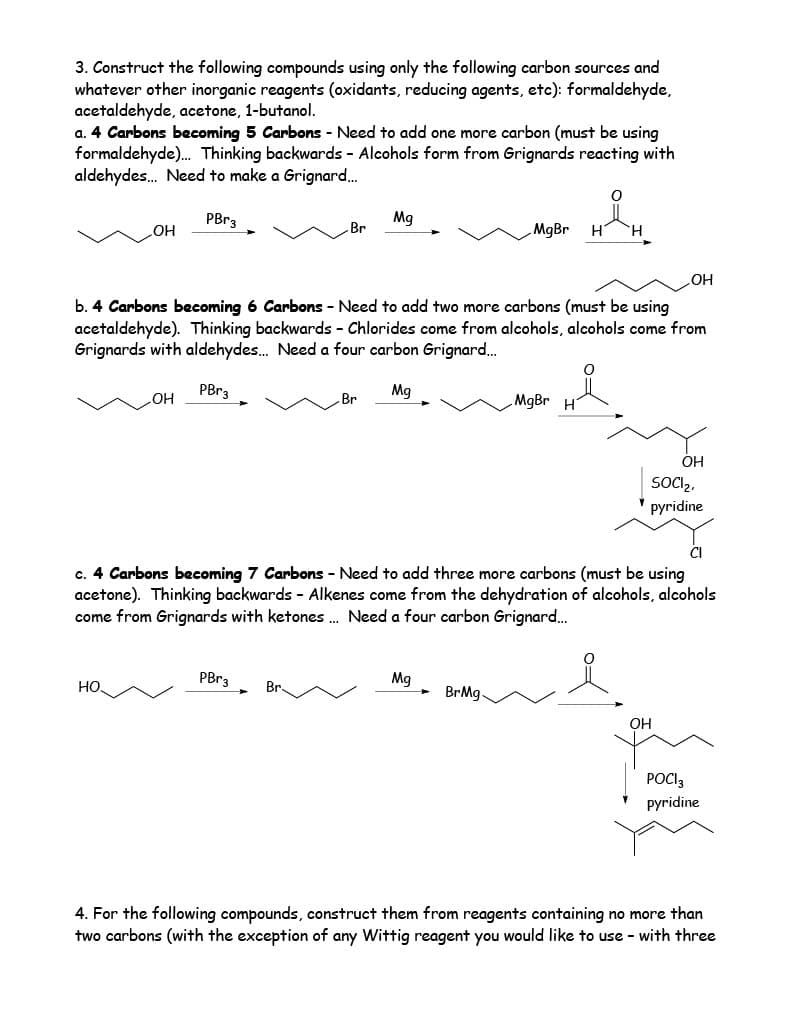
3. Construct the following compounds using only the following carbon sources and whatever other inorganic reagents (oxidants, reducing agents, etc): formaldehyde, acetaldehyde, acetone, 1-butanol. a. 4 Carbons becoming 5 Carbons - Need to add one more carbon (must be using formaldehyde)... Thinking backwards - Alcohols form from Grignards reacting with aldehydes... Need to make a Grignard... PBr3 OH НО. OH Br PBr3 Mg b. 4 Carbons becoming 6 Carbons - Need to add two more carbons (must be using acetaldehyde). Thinking backwards - Chlorides come from alcohols, alcohols come from Grignards with aldehydes... Need a four carbon Grignard.... PBr3 Mg Br MgBr H Mg BrMg- MgBr O H c. 4 Carbons becoming 7 Carbons - Need to add three more carbons (must be using acetone). Thinking backwards - Alkenes come from the dehydration of alcohols, alcohols come from Grignards with ketones... Need a four carbon Grignard... OH OH SOCI₂. pyridine OH Y CI POCI 3 pyridine 4. For the following compounds, construct them from reagents containing no more than two carbons (with the exception of any Wittig reagent you would like to use with three
Reactions of Ethers
Ethers (R-O-R’) are compounds formed by replacing hydrogen atoms of an alcohol (R-OH compound) or a phenol (C6H5OH) by an aryl/ acyl group (functional group after removing single hydrogen from an aromatic ring). In this section, reaction, preparation and behavior of ethers are discussed in the context of organic chemistry.
Epoxides
Epoxides are a special class of cyclic ethers which are an important functional group in organic chemistry and generate reactive centers due to their unusual high reactivity. Due to their high reactivity, epoxides are considered to be toxic and mutagenic.
Williamson Ether Synthesis
An organic reaction in which an organohalide and a deprotonated alcohol forms ether is known as Williamson ether synthesis. Alexander Williamson developed the Williamson ether synthesis in 1850. The formation of ether in this synthesis is an SN2 reaction.
Could you show the mechanism for Question 3, parts A-C?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









