Please explain question 1 and 8 1. How many degrees of unsaturation are present in your unknown monosubstituted benzene starting material? 8. Based on all of the data provided, what is the identity of the “G” group? (Please NEATLY & CLEARLY draw its full Lewis structure in the space provided) Thank you!
Please explain question 1 and 8 1. How many degrees of unsaturation are present in your unknown monosubstituted benzene starting material? 8. Based on all of the data provided, what is the identity of the “G” group? (Please NEATLY & CLEARLY draw its full Lewis structure in the space provided) Thank you!
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.59P
Related questions
Question
Please explain question 1 and 8
1. How many degrees of unsaturation are present in your unknown monosubstituted benzene starting material?
8. Based on all of the data provided, what is the identity of the “G” group? (Please NEATLY & CLEARLY draw its full Lewis structure in the space provided)
Thank you!
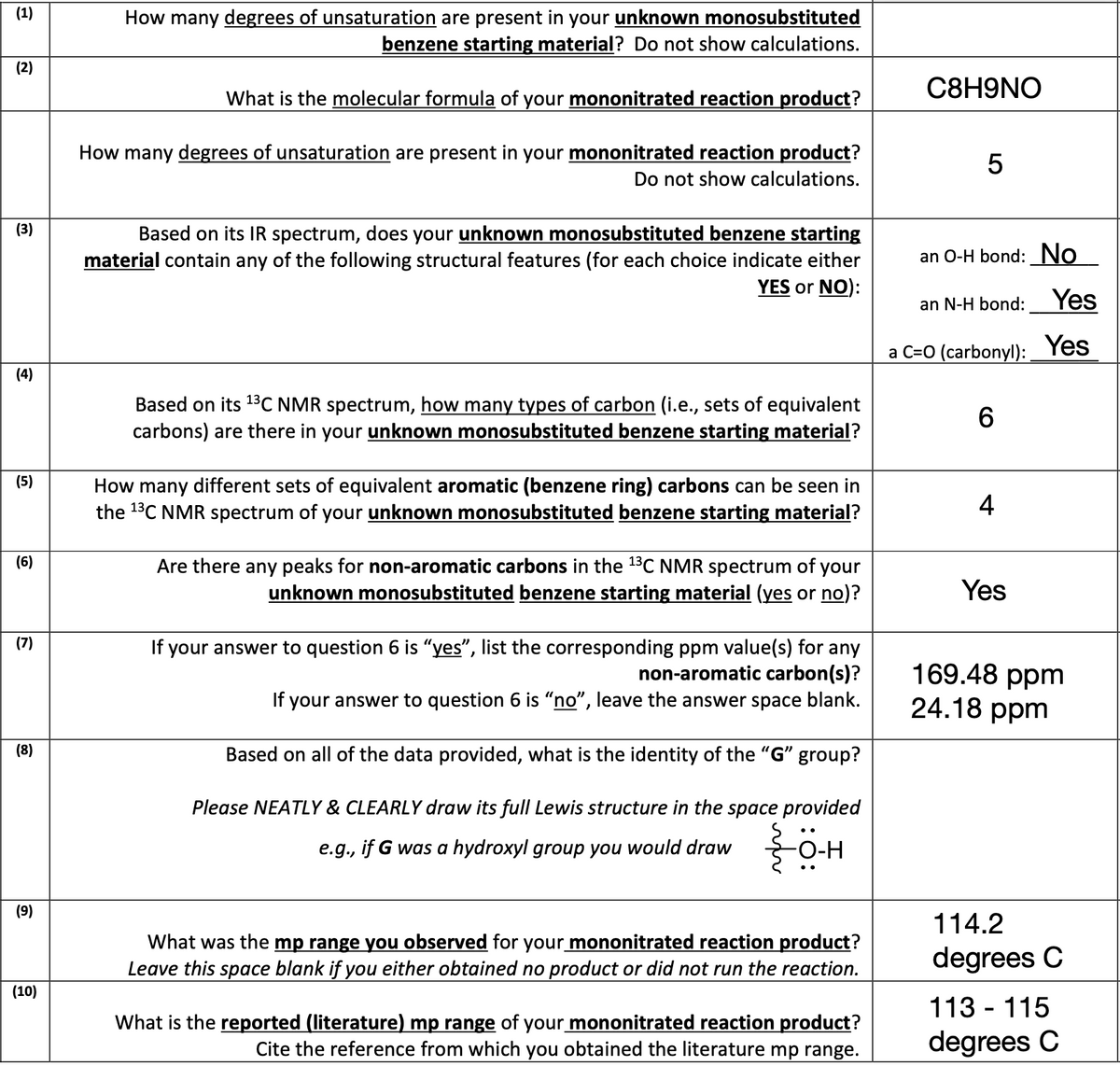
Transcribed Image Text:(1)
(2)
3
(4)
(5)
(6)
(7)
(8)
(9)
(10)
How many degrees of unsaturation are present in your unknown monosubstituted
benzene starting material? Do not show calculations.
What is the molecular formula of your mononitrated reaction product?
How many degrees of unsaturation are present in your mononitrated reaction product?
Do not show calculations.
Based on its IR spectrum, does your unknown monosubstituted benzene starting
material contain any of the following structural features (for each choice indicate either
YES or NO):
Based on its ¹³C NMR spectrum, how many types of carbon (i.e., sets of equivalent
carbons) are there in your unknown monosubstituted benzene starting material?
How many different sets of equivalent aromatic (benzene ring) carbons can be seen in
the ¹³C NMR spectrum of your unknown monosubstituted benzene starting material?
Are there any peaks for non-aromatic carbons in the ¹³C NMR spectrum of your
unknown monosubstituted benzene starting material (yes or no)?
If your answer to question 6 is "yes", list the corresponding ppm value(s) for any
non-aromatic carbon(s)?
If your answer to question 6 is "no", leave the answer space blank.
Based on all of the data provided, what is the identity of the "G" group?
Please NEATLY & CLEARLY draw its full Lewis structure in the space provided
e.g., if G was a hydroxyl group you would draw
-O-H
What was the mp range you observed for your mononitrated reaction product?
Leave this space blank if you either obtained no product or did not run the reaction.
What is the reported (literature) mp range of your mononitrated reaction product?
Cite the reference from which you obtained the literature mp range.
C8H9NO
5
an O-H bond: No
an N-H bond: Yes
a C=O (carbonyl):
Yes
6
4
Yes
169.48 ppm
24.18 ppm
114.2
degrees C
113-115
degrees C
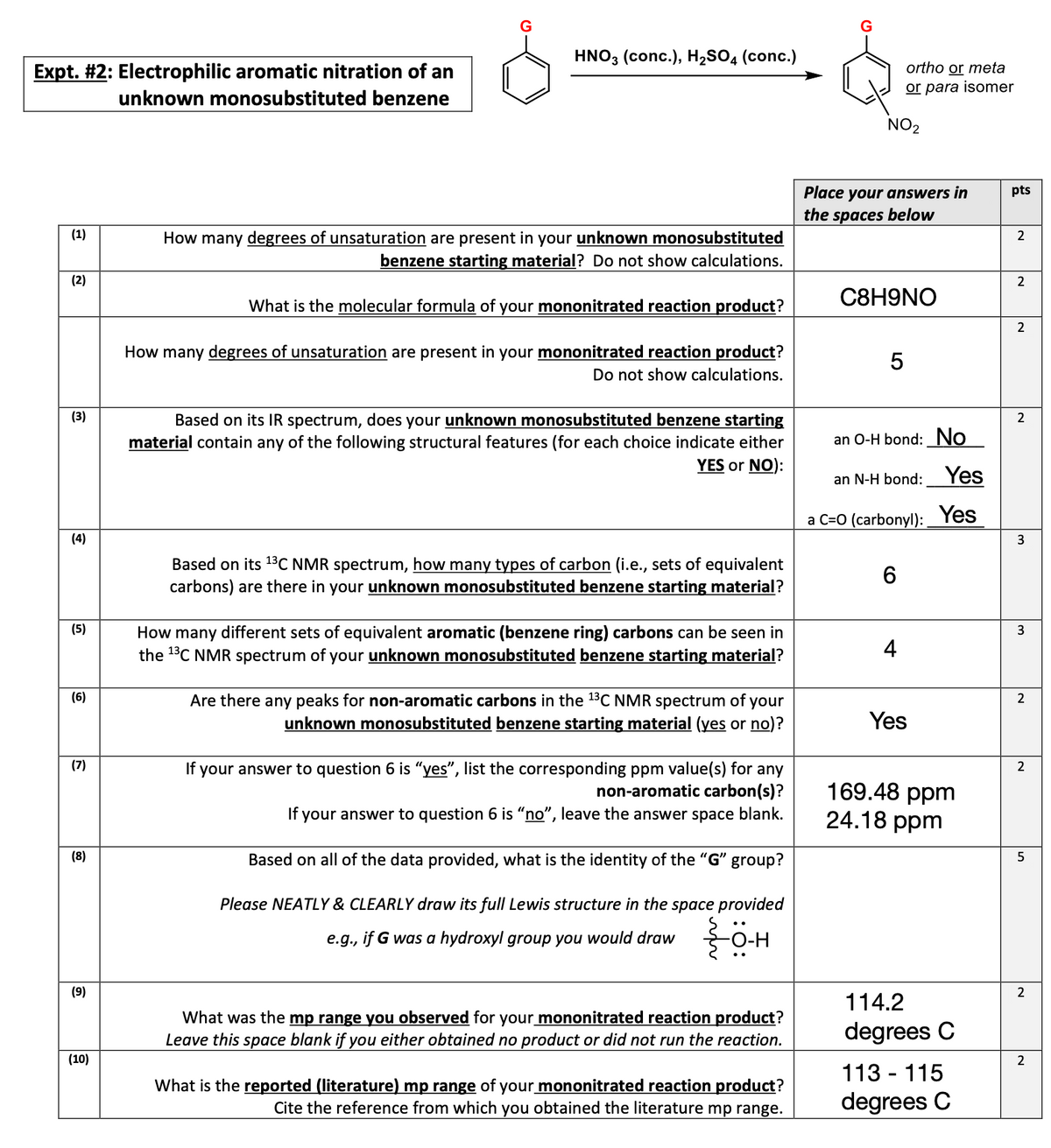
Transcribed Image Text:Expt. #2: Electrophilic aromatic nitration of an
unknown monosubstituted benzene
(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
G
HNO3 (conc.), H₂SO4 (conc.)
How many degrees of unsaturation are present in your unknown monosubstituted
benzene starting material? Do not show calculations.
What is the molecular formula of your mononitrated reaction product?
How many degrees of unsaturation are present in your mononitrated reaction product?
Do not show calculations.
Based on its IR spectrum, does your unknown monosubstituted benzene starting
material contain any of the following structural features (for each choice indicate either
YES or NO):
Based on its ¹³C NMR spectrum, how many types of carbon (i.e., sets of equivalent
carbons) are there in your unknown monosubstituted benzene starting material?
How many different sets of equivalent aromatic (benzene ring) carbons can be seen in
the ¹³C NMR spectrum of your unknown monosubstituted benzene starting material?
Are there any peaks for non-aromatic carbons in the ¹³C NMR spectrum of your
unknown monosubstituted benzene starting material (yes or no)?
If your answer to question 6 is "yes", list the corresponding ppm value(s) for any
non-aromatic carbon(s)?
If your answer to question 6 is "no", leave the answer space blank.
Based on all of the data provided, what is the identity of the "G" group?
Please NEATLY & CLEARLY draw its full Lewis structure in the space provided
e.g., if G was a hydroxyl group you would draw {-0-H
What was the mp range you observed for your mononitrated reaction product?
Leave this space blank if you either obtained no product or did not run the reaction.
What is the reported (literature) mp range of your mononitrated reaction product?
Cite the reference from which you obtained the literature mp range.
G
NO₂
ortho or meta
or para isomer
Place your answers in
the spaces below
C8H9NO
5
an O-H bond: No
an N-H bond: Yes
a C=O (carbonyl): Yes
6
Yes
169.48 ppm
24.18 ppm
114.2
degrees C
113-115
degrees C
pts
2
2
2
2
3
3
2
2
5
2
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
