3. Ethanol Production. Ethylenc gas, C2H4(g) and steam at 320°C and 1 atm pressure are fed to a reaction process as an equimolar mixture. The reaction goes by: C,H4(g) + H20G) → CzH;0H) The process goes to completion and produces 1 mol of liquid ethanol C,H50Hy that leaves the reactor at 25°C. What is the heat, Q (kJ), associated with the process per mole of ethanol produced? Note: You must provide a full material balance for the process for full credit. Note: You must draw a hypothetical path for the process for full credit. LP Data from Appendix C R C2H4(g) H20(g) C2H50H(9) A 1.424 3.470 3.518 14.394 X 103 1.450 X 103 20.001 X 10-3 -4.392 X 10 -6.002 X 10-6 0.121 X 10
3. Ethanol Production. Ethylenc gas, C2H4(g) and steam at 320°C and 1 atm pressure are fed to a reaction process as an equimolar mixture. The reaction goes by: C,H4(g) + H20G) → CzH;0H) The process goes to completion and produces 1 mol of liquid ethanol C,H50Hy that leaves the reactor at 25°C. What is the heat, Q (kJ), associated with the process per mole of ethanol produced? Note: You must provide a full material balance for the process for full credit. Note: You must draw a hypothetical path for the process for full credit. LP Data from Appendix C R C2H4(g) H20(g) C2H50H(9) A 1.424 3.470 3.518 14.394 X 103 1.450 X 103 20.001 X 10-3 -4.392 X 10 -6.002 X 10-6 0.121 X 10
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 22P
Related questions
Question
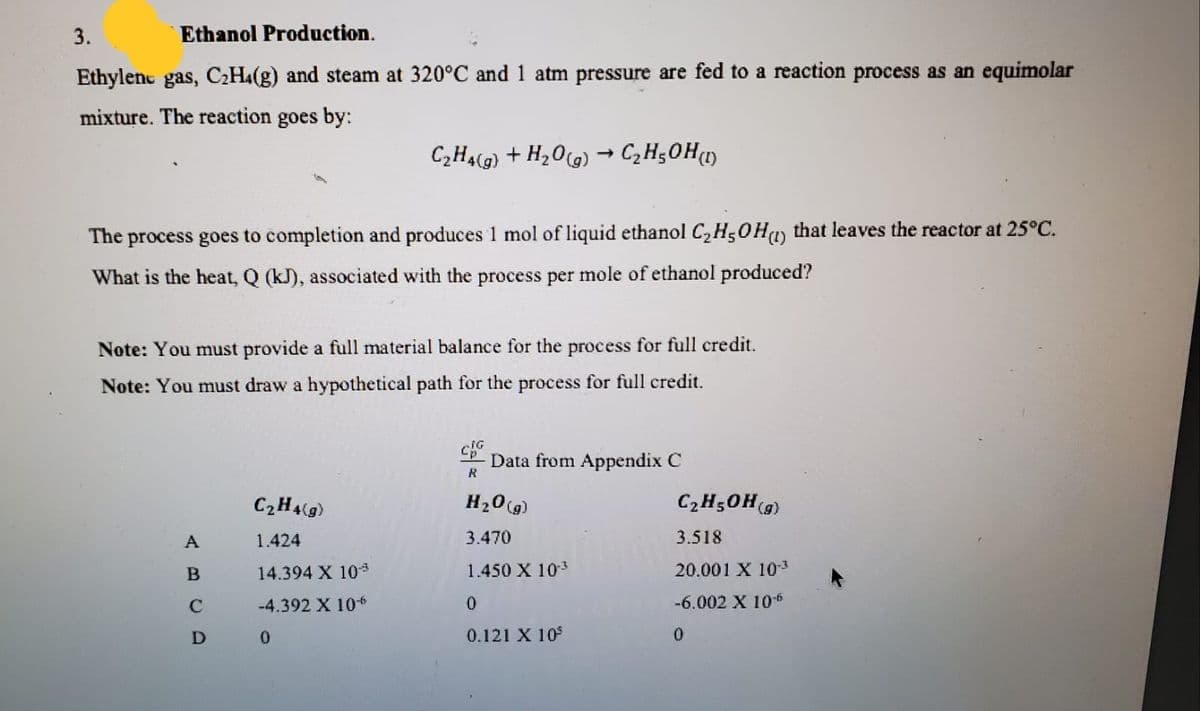
Transcribed Image Text:3.
Ethanol Production.
Ethylenc gas, C2H4(g) and steam at 320°C and 1 atm pressure are fed to a reaction process as an equimolar
mixture. The reaction goes by:
C,H4(g) + H20G) → CzH;0H)
The process goes to completion and produces 1 mol of liquid ethanol C,H50Hy that leaves the reactor at 25°C.
What is the heat, Q (kJ), associated with the process per mole of ethanol produced?
Note: You must provide a full material balance for the process for full credit.
Note: You must draw a hypothetical path for the process for full credit.
LP Data from Appendix C
R
C2H4(g)
H20(g)
C2H50H(9)
A
1.424
3.470
3.518
14.394 X 103
1.450 X 103
20.001 X 10-3
-4.392 X 10
-6.002 X 10-6
0.121 X 10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT