Benzoic acid, C;HsO:H, is typically used as a çalibrant for determining the specific heat of bomb calorimeters. It combusts via the following unbalanced chemical reaction: C,H5O2H (s) + 02(g) → CO2 (g) + H2O (1) 3. For this experimental setup and reaction, the constant volume heat flow is known to be gy = -26.979 kJ. Over the course of the reaction, the temperature rose from 19.863 °C to 22.540 °C. Calculate the heat capacity, Ceu, for this calorimeter in kJ °C-.
Benzoic acid, C;HsO:H, is typically used as a çalibrant for determining the specific heat of bomb calorimeters. It combusts via the following unbalanced chemical reaction: C,H5O2H (s) + 02(g) → CO2 (g) + H2O (1) 3. For this experimental setup and reaction, the constant volume heat flow is known to be gy = -26.979 kJ. Over the course of the reaction, the temperature rose from 19.863 °C to 22.540 °C. Calculate the heat capacity, Ceu, for this calorimeter in kJ °C-.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.83E: Benzoic acid, C6H5COOH, is a common standard used in bomb calorimeters, which maintain a constant...
Related questions
Question
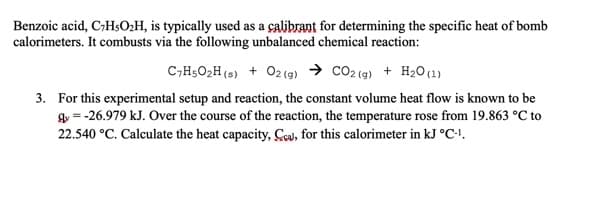
Transcribed Image Text:Benzoic acid, C;HsO:H, is typically used as a çalibrant for determining the specific heat of bomb
calorimeters. It combusts via the following unbalanced chemical reaction:
C,H5O2H (s) + 02(g) → CO2 (g) + H2O(1)
3. For this experimental setup and reaction, the constant volume heat flow is known to be
gy = -26.979 kJ. Over the course of the reaction, the temperature rose from 19.863 °C to
22.540 °C. Calculate the heat capacity, Cou, for this calorimeter in kJ °C-!.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning