3. Find the molar absorptivity Concentration Measured coefficient, the R? assuming that the path length is 1 cm and the equation of the data. (give your (ppm) absorbance 0.01 0.0715 0.03 0.2986 0.05 0.4995 answer rounded off to two 0.07 0.5563 0.1 0.794 decimal place with complete units). 0.12 0.9521 0.14 1.1096 0.16 1.3864 0.18 1.4221 0.2 1.5163 0.23 1.7039 0.25 0.27 1.9034 2.2484
3. Find the molar absorptivity Concentration Measured coefficient, the R? assuming that the path length is 1 cm and the equation of the data. (give your (ppm) absorbance 0.01 0.0715 0.03 0.2986 0.05 0.4995 answer rounded off to two 0.07 0.5563 0.1 0.794 decimal place with complete units). 0.12 0.9521 0.14 1.1096 0.16 1.3864 0.18 1.4221 0.2 1.5163 0.23 1.7039 0.25 0.27 1.9034 2.2484
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.16QAP
Related questions
Question
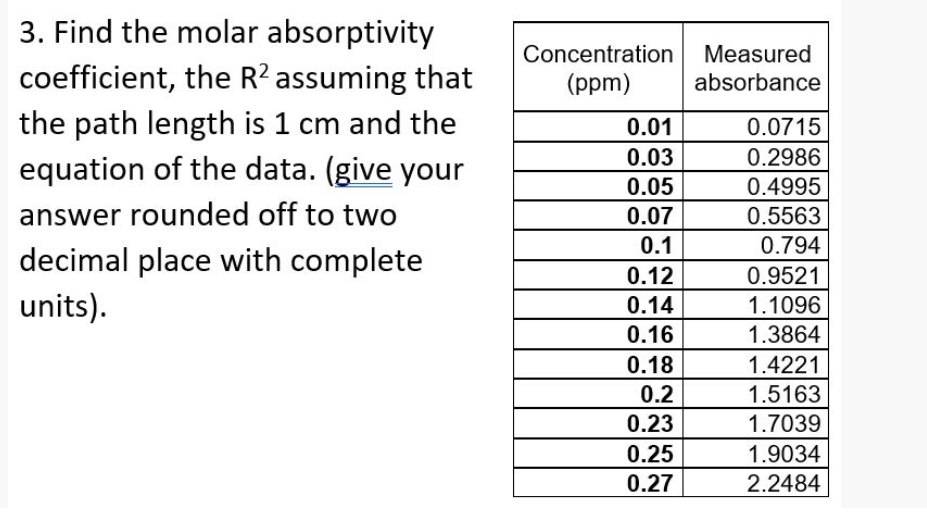
Transcribed Image Text:3. Find the molar absorptivity
coefficient, the R? assuming that
Concentration Measured
(ppm)
absorbance
the path length is 1 cm and the
equation of the data. (give your
0.01
0.0715
0.03
0.2986
0.05
0.4995
answer rounded off to two
0.07
0.5563
0.1
0.794
decimal place with complete
0.12
0.9521
units).
0.14
1.1096
0.16
1.3864
0.18
1.4221
0.2
1.5163
0.23
1.7039
0.25
0.27
1.9034
2.2484
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning