Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 21ALQ: True or false? The atom with the largest subscript in a formula is the atom with the largest percent...
Related questions
Question
100%
I uploaded photos
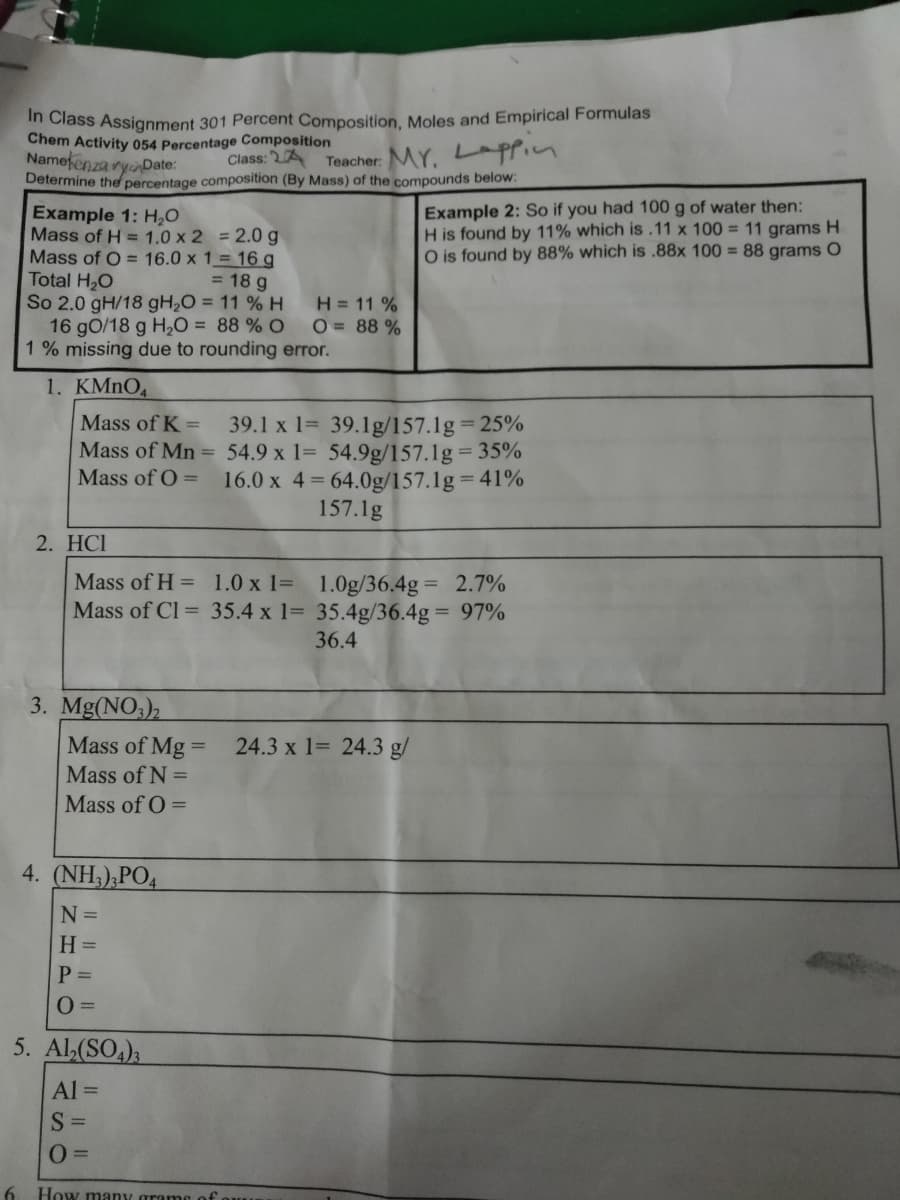
Transcribed Image Text:in Class Assignment 301 Percent Composition, Moles and Empirical Formulas
Chem Activity 054 Percentage Composition
Namefenza ryinDate:
Determine the percentage composition (By Mass) of the compounds below:
Class: 2A
Teacher: MY. Lppin
Example 2: So if you had 100 g of water then:
H is found by 11% which is.11 x 100 = 11 grams H
O is found by 88% which is .88x 100 = 88 grams O
Example 1: H,0
Mass of H = 1.0 x 2 = 2.0 g
Mass of O = 16.0 x 1= 16 g
Total H,O
So 2.0 gH/18 gH,O = 11 % H
16 gO/18 g H2O = 88 % O
1 % missing due to rounding error.
= 18 g
H = 11 %
O = 88 %
1. KMNO,
Mass of K =
39.1 x 1= 39.1g/157.1g= 25%
Mass of Mn = 54.9 x 1= 54.9g/157.1g 35%
Mass of O =
16.0 x 4= 64.0g/157.1g= 41%
157.1g
2. HCI
Mass of H = 1.0 x 1= 1.0g/36.4g = 2.7%
Mass of Cl = 35.4 x 1= 35.4g/36.4g = 97%
36.4
3. Mg(NO3),
Mass of Mg =
24.3 x 1= 24.3 g/
Mass of N =
Mass of O =
4. (NH;),PO4
N =
H =
P =
5. Al,(SO,),
Al =
S =
How many gran

Transcribed Image Text:8. How much silver can be produced from 125 g of Ag,S?
Chem Activity 055 Determining Empirical Formulas
What is the empirical formula (lowest whole number ratio) of the compounds below?
Example 1: 11% H, 88% O
Percent grams divide by molar mass = Moles
Divide each by smaller
= H %LL
88% O = *88 g O /
1 g/mol
16g/mol
= 11 moles/5.5 = 2
= 5.5 moles/5.5 = 1
mole Ratio
2 parts H to 1 part O so
*11 g H I
*Assume that 100 % is 100 grams
Example 2: 30% O, 70% Fe
Percent grams divide by molar mass = Moles
30% O, so 30 g0 16 g/mol
70% Fe
Divide each by smaller. If ratio not close to whole then double
mole Ratio
= 1.875/1.25 = 1,5 moles x 2 = 3
70g Fe / 55.8 g/mol = 1.25/1.25 = 1 moles x 2 = 2
2 parts Fe to 3 part O so
1. 75% carbon, 25% hydrogen
Percent
grams divide by molar mass = Moles
Divide each by smaller
H %S
25 g H /
75g C/
1 g/mol
12g/mol
= 25 moles/6.25 = 4
= 6.25 moles/6.25 = 1
mole Ratio
4 parts H to 1 part C so CH
75% C
2. 52.7% potassium, 47.3% chlorine
3. 22.1% aluminum, 25.4% phosphorus, 52.5% oxygen
4. 13% magnesium, 87% bromine
5. 32.4% sodium, 22.5 % Sulfur, 45.1 % oxygen.
6. 25.3% copper, 12.9% sulfur, 25.7% oxygen, 36.1 % water
7.22.3 % oxygen, 77.7 % iron
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax