Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 29CTQ
Related questions
Question
I need help please
(Not honor class)
(Not grading)
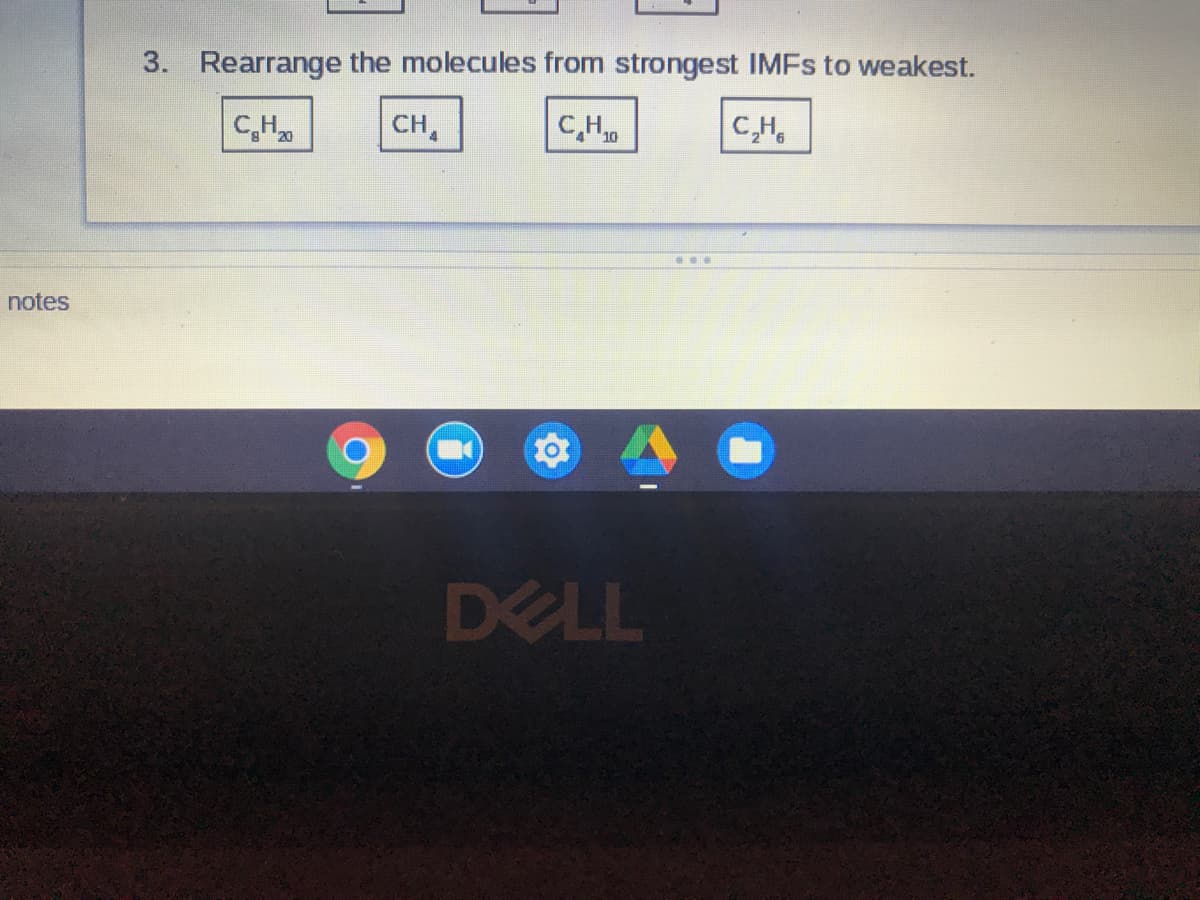
Transcribed Image Text:3. Rearrange the molecules from strongest IMFS to weakest.
CH0
C,H,
CH
C,H
...
notes
DELL
Expert Solution
Step 1
Answer:-
This question is answered by using the simple concept of intermolecular forces (IMF) of alkanes. In alkanes the strength of intermolecular forces increases with increase in the number of carbon atoms because molecular mass increases.
The correct order of IMF from strongest to weakest is given as follows,
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning