3. Select the correct statement from the following choices that best explain the result recorded in the table as shown in question 1. O A The ratio of the effusion rate of hydrogen gas to that of methane gas is 3.20 B The molar mass obtained from experiment is lower than that of the actual molar mass of the gas A C. The molar mass obtained is different from the actual motar mass of Gas A due to the presence of impurities O D. The impurities presence might have the molar mass that is smaller than Gas A 4. What difference would you expect on the time of effusion and molar mass of gas Aif the experiment wvere carried out using the same apparatus at a higher temperature. A. At a higher temperature, gas molecules move inore rapidiy and effusion time would therefore longer B. At high temperature, the molar mass of gas A obtaned would be lower CAt high temperature, the ratio of effusion rate of hydrogen to gas A would be higher 0 At higher temperature, there is no change on the effusion rate of hydrogen gas to that of gas A
3. Select the correct statement from the following choices that best explain the result recorded in the table as shown in question 1. O A The ratio of the effusion rate of hydrogen gas to that of methane gas is 3.20 B The molar mass obtained from experiment is lower than that of the actual molar mass of the gas A C. The molar mass obtained is different from the actual motar mass of Gas A due to the presence of impurities O D. The impurities presence might have the molar mass that is smaller than Gas A 4. What difference would you expect on the time of effusion and molar mass of gas Aif the experiment wvere carried out using the same apparatus at a higher temperature. A. At a higher temperature, gas molecules move inore rapidiy and effusion time would therefore longer B. At high temperature, the molar mass of gas A obtaned would be lower CAt high temperature, the ratio of effusion rate of hydrogen to gas A would be higher 0 At higher temperature, there is no change on the effusion rate of hydrogen gas to that of gas A
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 27QAP: The gas in the discharge cell of a laser contains (in mole percent) 11% CO2, 5.3% N2, and 84% He....
Related questions
Question
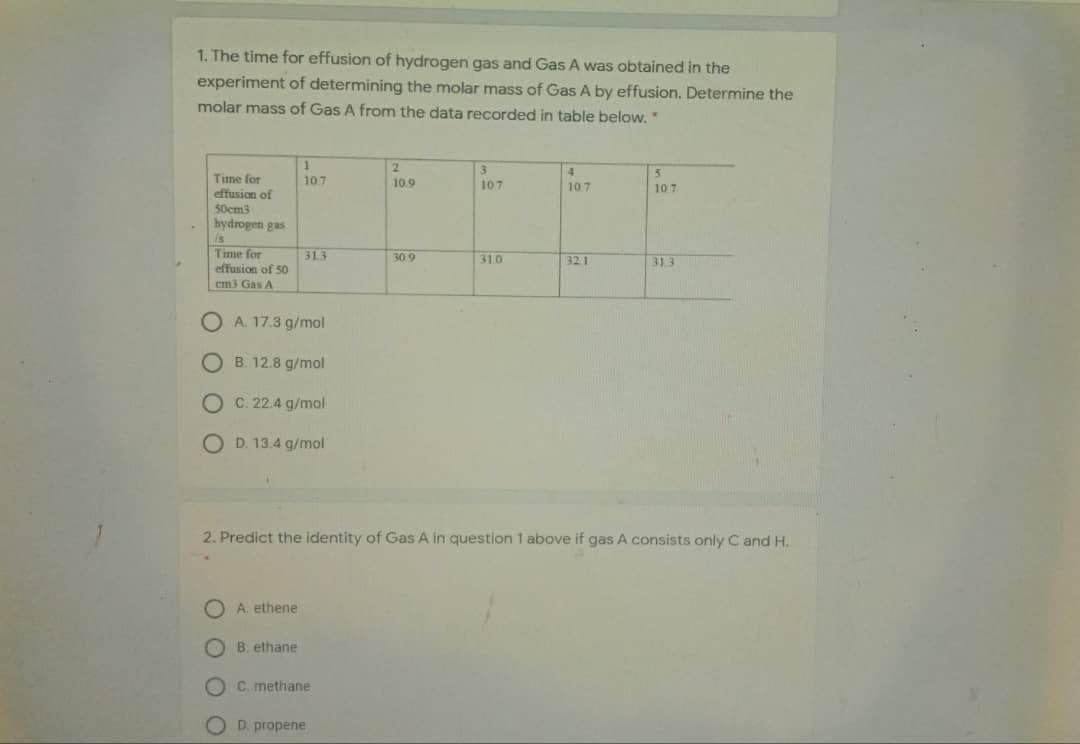
Transcribed Image Text:1. The time for effusion of hydrogen gas and Gas A was obtained in the
experiment of determining the molar mass of Gas A by effusion. Determine the
molar mass of Gas A from the data recorded in table below."
2.
4
Time for
effusion of
107
109
107
10.7
107
50cm3
hydrogen gas
is
Time for
effusion of 50
313
30 9
310
321
313
em3 Gas A
O A. 17.3 g/mol
O B. 12.8 g/mol
O C. 22.4 g/mol
O D. 13.4 g/mol
2. Predict the identity of Gas A in question 1 above if gas A consists only C and H.
O A. ethene
O B. ethane
O C. methane
D. propene
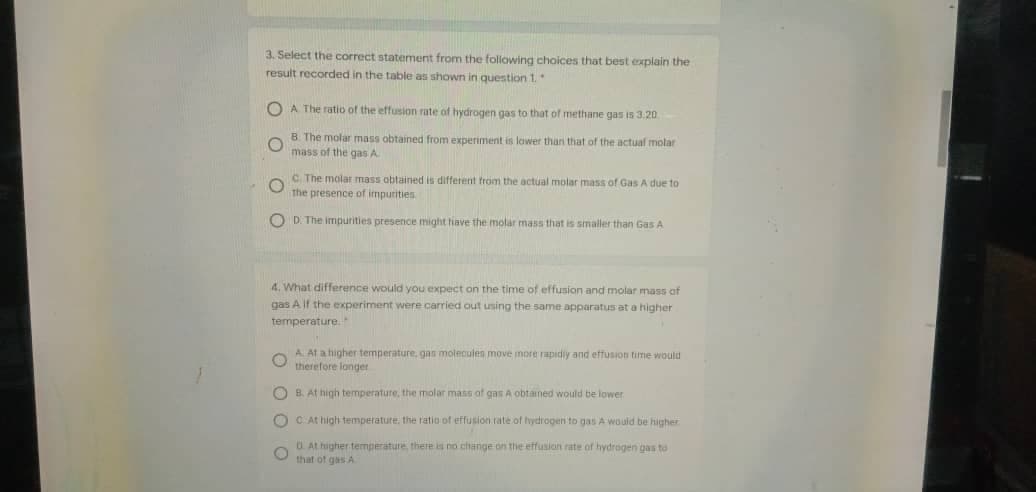
Transcribed Image Text:3. Select the correct statement from the following choices that best explain the
result recorded in the table as shown in question 1.
O A The ratio of the effusion rate of hydrogen gas to that of methane gas is 3:20
B The molar mass obtained from experiment
mass of the gas A
lawer than that of the actual molar
C The molar mass obtained is different from the actual molar mass of Gas A due to
The presence of impurities
O D. The imnpurities presence might tiave the molar mass that is smaller than Gas A
4. What difference would you expect on the time of effusion and molar mass of
gas A If the experiment were carried out using the same apparatus at a higher
temperature.
A. At a higher temperature, gan moleculen move more rapidiy and effusion time would
therefore longer
O B. At high temperature, the molar mass of gas A obtained would be lower
O C At high temperature. the ratio of effusion rate of hydrogen to gas A would be higher
O. At higher temperature, there is no change on thie effusion rate of hydrogen gas to
that of gas A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,