3. Suppose 12.7 g of C2H2 reacts with 17.3 g O2 according to the reaction below. What is the mass of CO2 produced? What is the limiting C2H2(9) + Ozıg) reagent? > CO29) + H2OM 4. Nitrogen monoxide is formed primarily in car engines, and it can react with oxygen to form gaseous nitrogen dioxide. Nitrogen dioxide forms nitric acid when it comes in contact with water, another component of acid rain. If 3.12 g of nitrogen monoxide reacts with 4.15 g of oxygen, how much nitrogen dioxide will form? What is the limiting reagent? Which reactant remains in excess, and in what mass? 2NO(g) + Oz(g) –→ 2NO2(g)
3. Suppose 12.7 g of C2H2 reacts with 17.3 g O2 according to the reaction below. What is the mass of CO2 produced? What is the limiting C2H2(9) + Ozıg) reagent? > CO29) + H2OM 4. Nitrogen monoxide is formed primarily in car engines, and it can react with oxygen to form gaseous nitrogen dioxide. Nitrogen dioxide forms nitric acid when it comes in contact with water, another component of acid rain. If 3.12 g of nitrogen monoxide reacts with 4.15 g of oxygen, how much nitrogen dioxide will form? What is the limiting reagent? Which reactant remains in excess, and in what mass? 2NO(g) + Oz(g) –→ 2NO2(g)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 20CR: Solid calcium carbide (CaC2)reacts with liquid water to produce acetylene gas (C2H2)and aqueous...
Related questions
Question
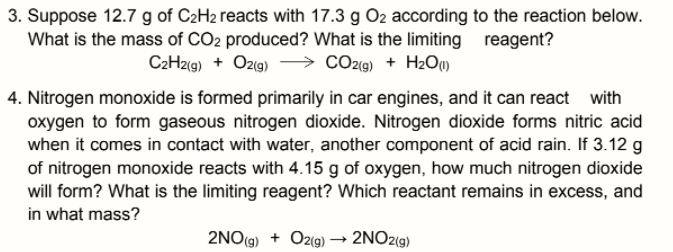
Transcribed Image Text:3. Suppose 12.7 g of C2H2 reacts with 17.3 g O2 according to the reaction below.
What is the mass of CO2 produced? What is the limiting
C2H2(9) + Ozıg)
reagent?
> CO29) + H2OM
4. Nitrogen monoxide is formed primarily in car engines, and it can react with
oxygen to form gaseous nitrogen dioxide. Nitrogen dioxide forms nitric acid
when it comes in contact with water, another component of acid rain. If 3.12 g
of nitrogen monoxide reacts with 4.15 g of oxygen, how much nitrogen dioxide
will form? What is the limiting reagent? Which reactant remains in excess, and
in what mass?
2NO(g) + Oz(g) –→ 2NO2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning