3. The following data for the first-order decomposition of penicillin are obtained from Swintosky et al. Temp (°C) Тemp (К) 1/Тemp (1/K) 1st-order k (hr') In k 54 327 3.06 х 10-3 0.119 - 2.129 43 316 3.16 x 10-3 0.0403 - 3.211 37 310 3.23 х 10-3 0.0216 - 3.835 a) Plot the results and calculate the activation energy (E,) and the Arrhenius factor (A). 1/T (K)
3. The following data for the first-order decomposition of penicillin are obtained from Swintosky et al. Temp (°C) Тemp (К) 1/Тemp (1/K) 1st-order k (hr') In k 54 327 3.06 х 10-3 0.119 - 2.129 43 316 3.16 x 10-3 0.0403 - 3.211 37 310 3.23 х 10-3 0.0216 - 3.835 a) Plot the results and calculate the activation energy (E,) and the Arrhenius factor (A). 1/T (K)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 87QAP: A drug decomposes in the blood by a first-order process. A pill containing 0.500 g of the active...
Related questions
Question
How did my teacher get the 1st order row and the ln k row
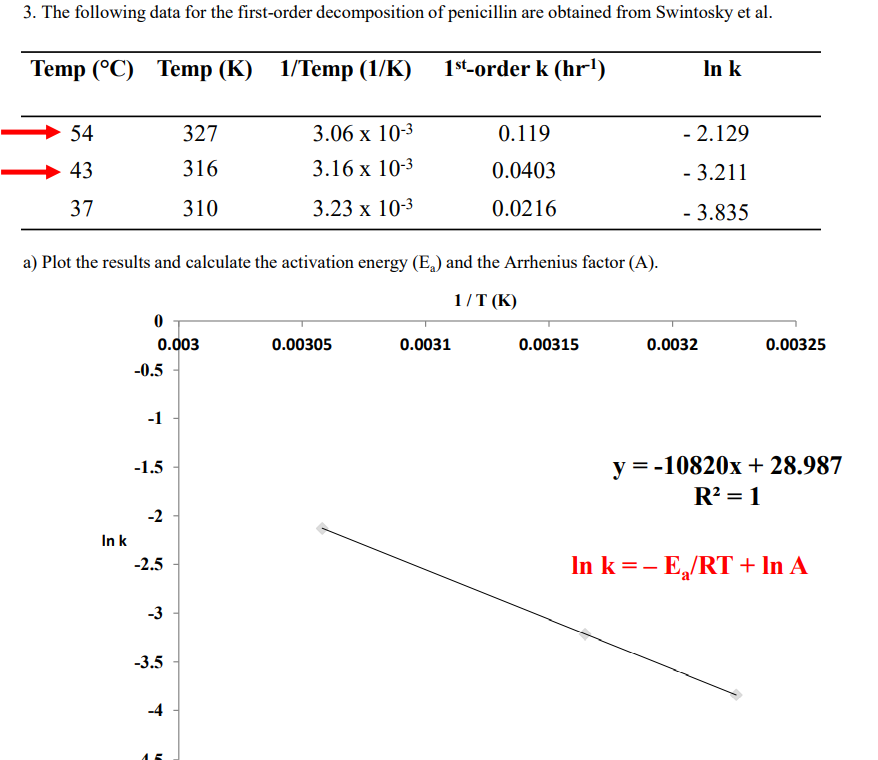
Transcribed Image Text:3. The following data for the first-order decomposition of penicillin are obtained from Swintosky et al.
Temp (°C) Temp (K) 1/Temp (1/K)
1st-order k (hr')
In k
54
327
3.06 х 103
0.119
- 2.129
43
316
3.16 х 10-3
0.0403
- 3.211
37
310
3.23 x 10-3
0.0216
- 3.835
a) Plot the results and calculate the activation energy (E,) and the Arrhenius factor (A).
1/T (K)
0.003
0.00305
0.0031
0.00315
0.0032
0.00325
-0.5
-1
-1.5
y = -10820x+ 28.987
R? = 1
-2
In k
In k =- E,/RT + In A
-2.5
-3
-3.5
-4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax