The first-order rate constant for the thermal decomposition of 3-methylcyclobutanone has the values T/K k/1045-1 T/K k/104s-1 552.24 0.4259 589.05 6.459 561.81 0.8936 596.96 11.201 570.41 1.707 606.14 20.83 579.35 3.207 a. Find the value of the activation energy and the value of the preexponential factor. b. Find the value of the rate constant at 600.0 K. c. Find the time for 80.0% of the reactant to react at 600.0 K.
The first-order rate constant for the thermal decomposition of 3-methylcyclobutanone has the values T/K k/1045-1 T/K k/104s-1 552.24 0.4259 589.05 6.459 561.81 0.8936 596.96 11.201 570.41 1.707 606.14 20.83 579.35 3.207 a. Find the value of the activation energy and the value of the preexponential factor. b. Find the value of the rate constant at 600.0 K. c. Find the time for 80.0% of the reactant to react at 600.0 K.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter13: Chemical Kinetics
Section: Chapter Questions
Problem 13.59QE
Related questions
Question
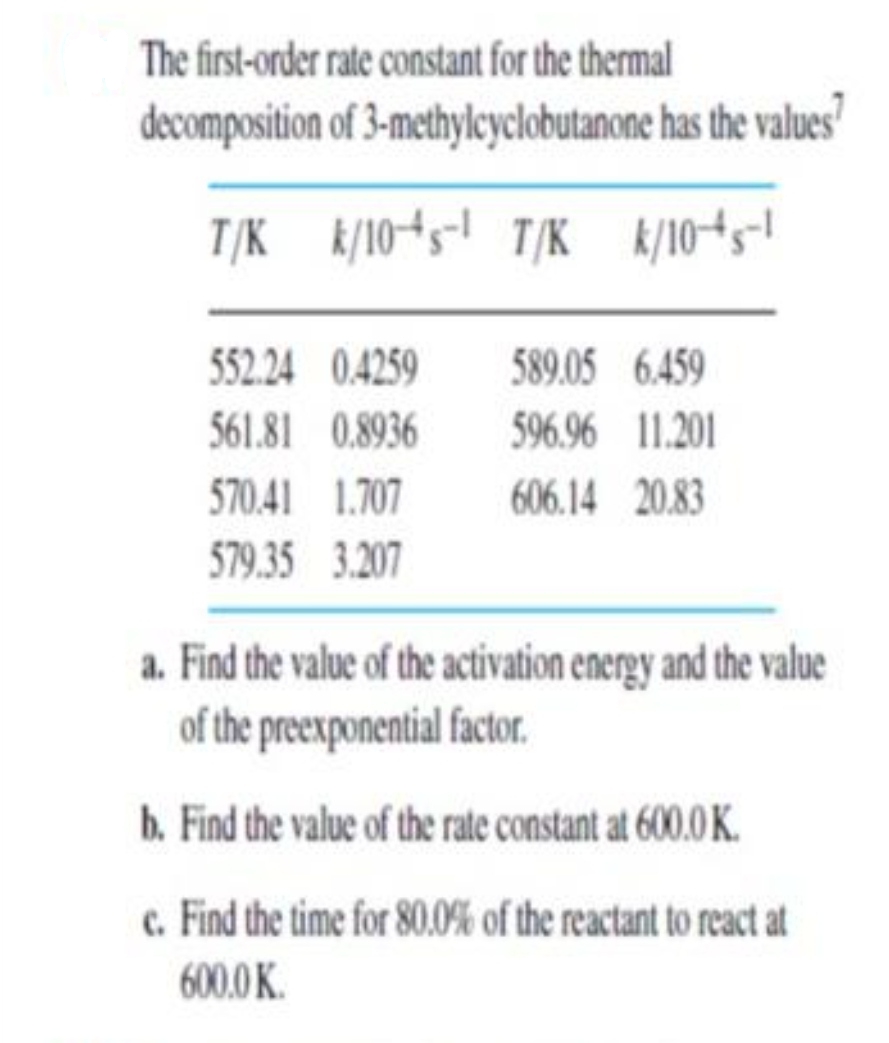
Transcribed Image Text:The first-order rate constant for the thermal
decomposition of 3-methylcyclobutanone has the values
T/K k/1045-! T/K k/104s!
552.24 0.4259
589.05 6.459
561.81 0.8936
596.96 11.201
570.41 1.707
606.14 20.83
579.35 3.207
a. Find the value of the activation energy and the value
of the preexponential factor.
b. Find the value of the rate constant at 600.0 K.
c. Find the time for 80.0% of the reactant to react at
600.0 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning