3. The following diagram shows an energy profile for an uncatalysed reaction. reactant products Reaction coordinate (a) Referring to the energy profile, state whether the reaction is exothermic or endothermic. Explain briefly. (bXi) In the above diagram, draw a labelled energy profile for the catalysed reaction. (i) Hence, explain why the catalyst can speed up the reaction ABaug
3. The following diagram shows an energy profile for an uncatalysed reaction. reactant products Reaction coordinate (a) Referring to the energy profile, state whether the reaction is exothermic or endothermic. Explain briefly. (bXi) In the above diagram, draw a labelled energy profile for the catalysed reaction. (i) Hence, explain why the catalyst can speed up the reaction ABaug
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 115SCQ: Methane, CH4, can be converted to methanol, which, like ethanol, can be used as a fuel. The energy...
Related questions
Question
Transcribed Image Text:Wondershare PDFelement Pro
檔案 編輯 檢視 工具 前往 窗口 說明
70% [4)
A АВС
週五下午8:31
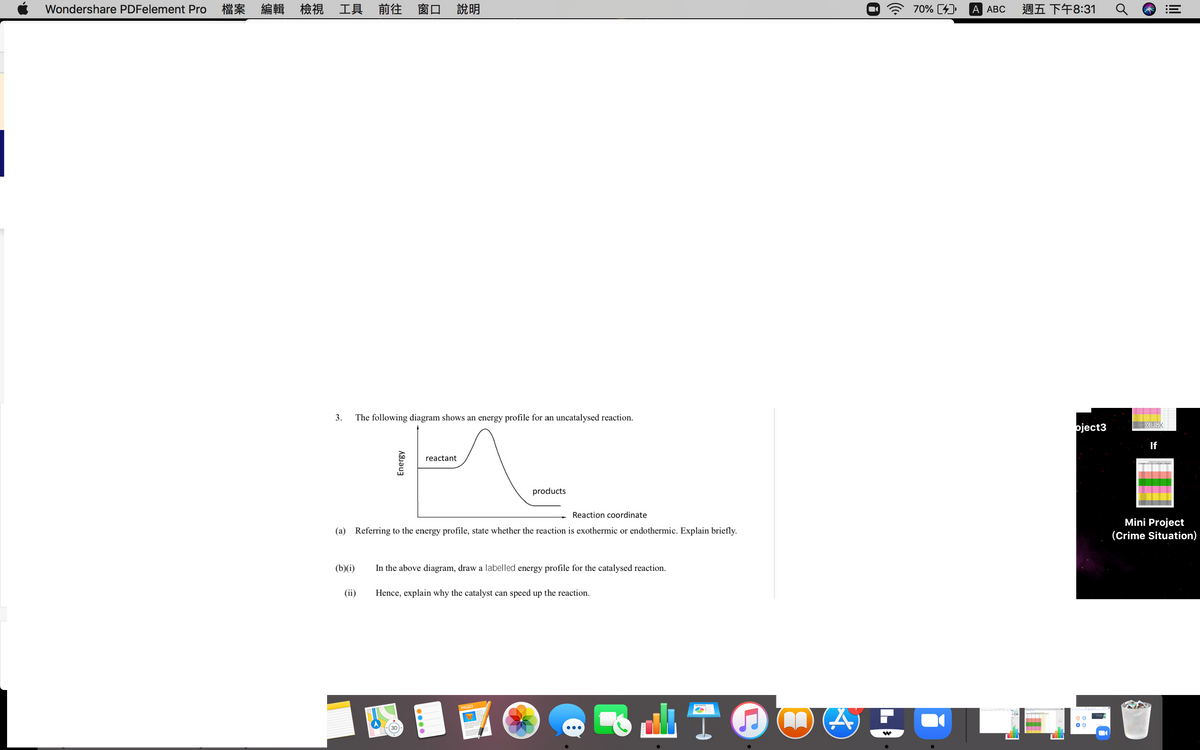
3.
The following diagram shows an energy profile for an uncatalysed reaction.
EXLSX
pject3
If
reactant
products
Reaction coordinate
Mini Project
(Crime Situation)
(a) Referring to the energy profile, state whether the reaction is exothermic or endothermic. Explain briefly.
(b)(i)
In the above diagram, draw a labelled energy profile for the catalysed reaction.
(ii)
Hence, explain why the catalyst can speed up the reaction.
PAGES
Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning