30. A saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92% (w/v) NaCl in water. What volume of the saline solution must be administered to the patient in order to deliver 7.7 g of NaCl? A) 4 mL of saline solution B) 7.1 mL of saline solution C) 840 mL of saline solution D) 140 mL of saline solution
30. A saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92% (w/v) NaCl in water. What volume of the saline solution must be administered to the patient in order to deliver 7.7 g of NaCl? A) 4 mL of saline solution B) 7.1 mL of saline solution C) 840 mL of saline solution D) 140 mL of saline solution
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 79E: D5W is a solution used as an intravenous fluid. It is a 5.0% by mass solution of dextrose (C6H12O6)...
Related questions
Question
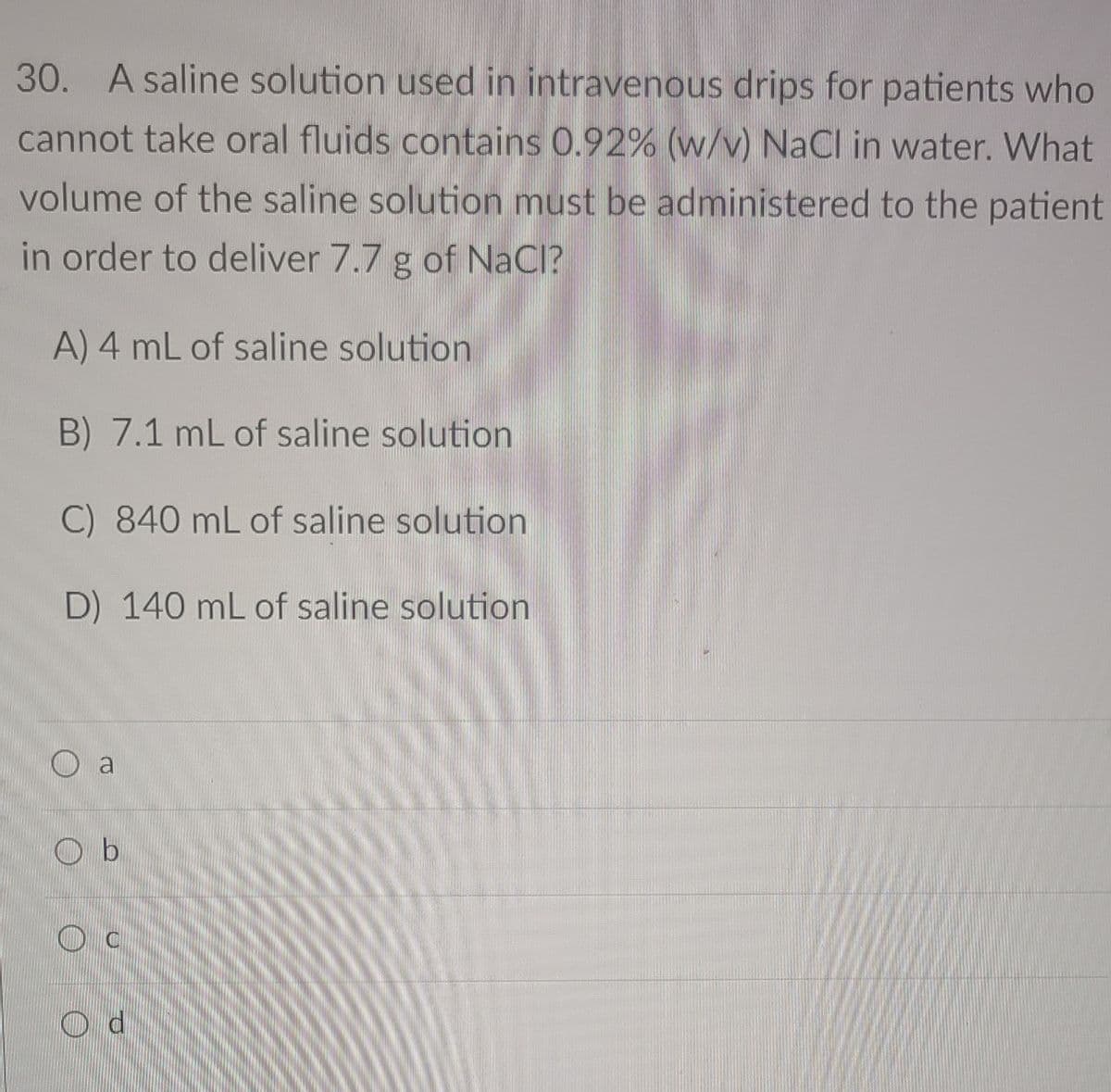
Transcribed Image Text:30. A saline solution used in intravenous drips for patients who
cannot take oral fluids contains 0.92% (w/v) NaCl in water. What
volume of the saline solution must be administered to the patient
in order to deliver 7.7 g of NaCl?
A) 4 mL of saline solution
B) 7.1 mL of saline solution
C) 840 mL of saline solution
D) 140 mL of saline solution
O a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning