concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copp copper(1 Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical Fe(s) + CuSO4(aq) → Cu(s) + FeSO ɖ(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way powdered iron to a 300. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then and weighs the precipitate, and finds that it has a mass of 125. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number digits. ?
concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copp copper(1 Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical Fe(s) + CuSO4(aq) → Cu(s) + FeSO ɖ(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way powdered iron to a 300. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then and weighs the precipitate, and finds that it has a mass of 125. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number digits. ?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 75QAP: A student is given 0.930 g of an unknown acid, which can be either oxalic acid, H2C2O4, or citric...
Related questions
Question
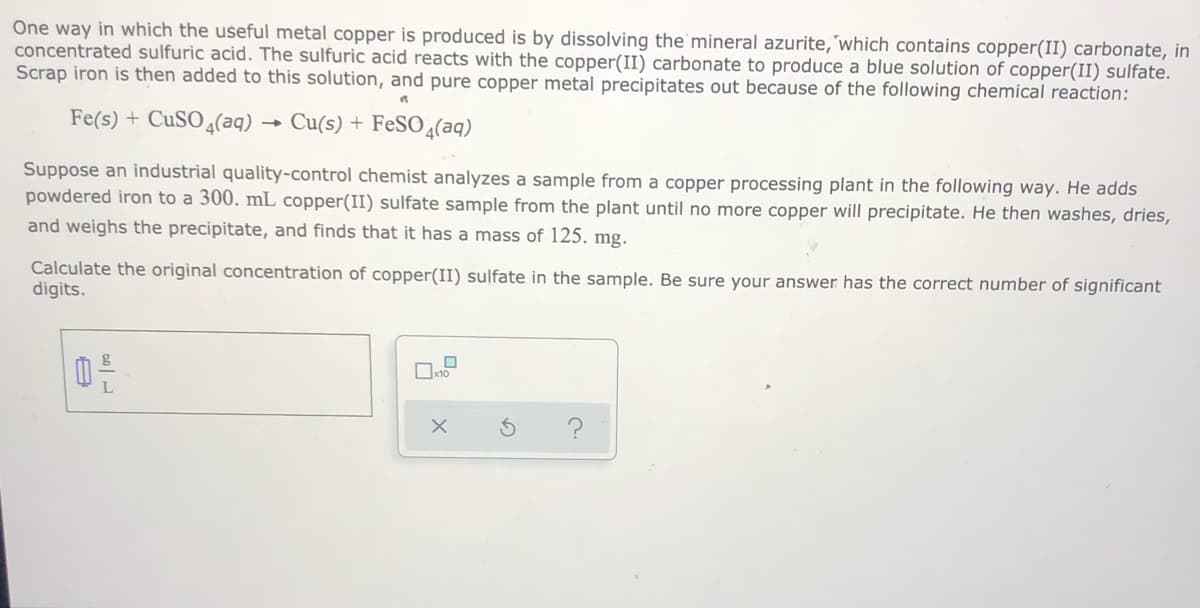
Transcribed Image Text:One way in which the useful metal copper is produced is by dissolving the mineral azurite,'which contains copper(II) carbonate, in
concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate.
Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction:
Fe(s) + CUSO4(aq)
Cu(s) + FeSO 4(aq)
Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds
powdered iron to a 300. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries,
and weighs the precipitate, and finds that it has a mass of 125. mg.
Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant
digits.
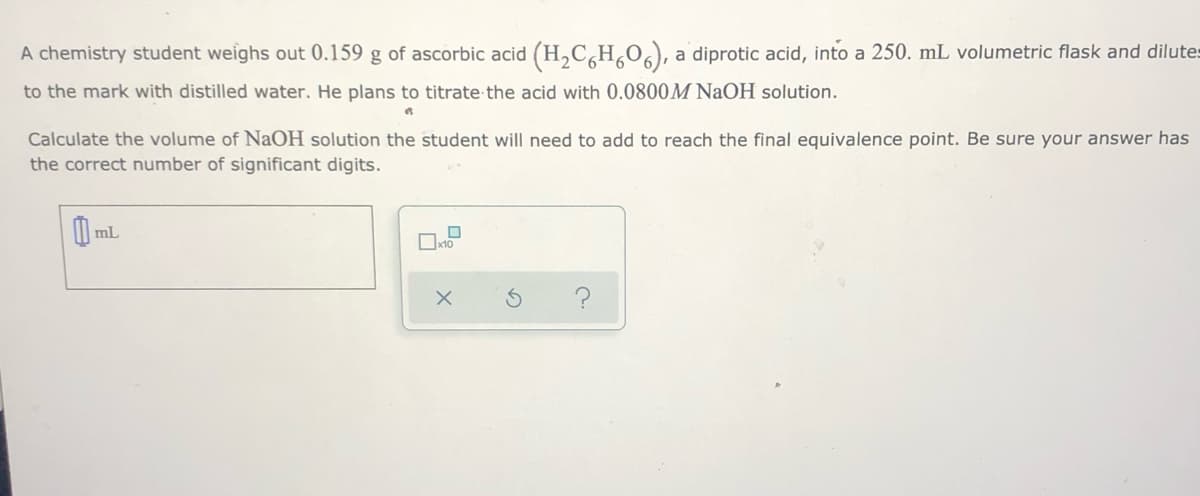
Transcribed Image Text:A chemistry student weighs out 0.159 g of ascorbic acid (H,C,H,0,), a diprotic acid, into a 250. mL volumetric flask and dilutes
to the mark with distilled water. He plans to titrate the acid with 0.0800M NaOH solution.
Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has
the correct number of significant digits.
mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax