33 34 = 35 = 36 37 38 39 40 = 41 = 42 = 43 = 44 Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H,0). If 14.3 g of sodium chloride is produced from the reaction of 24.1g of hydrochloric acid and 12.4 g of sodium hydroxide, calculate the percent yleld of sodium chloride. Round your answer to 3 significant figures. % Submit Assignment 2021 McGraw Hill LLC. AN Rights Reserved Terms of Use 1 Prvcy Center Accessibity
33 34 = 35 = 36 37 38 39 40 = 41 = 42 = 43 = 44 Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H,0). If 14.3 g of sodium chloride is produced from the reaction of 24.1g of hydrochloric acid and 12.4 g of sodium hydroxide, calculate the percent yleld of sodium chloride. Round your answer to 3 significant figures. % Submit Assignment 2021 McGraw Hill LLC. AN Rights Reserved Terms of Use 1 Prvcy Center Accessibity
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 47A
Related questions
Question
100%
Transcribed Image Text:33
34
= 35
=36
37
38
39
40
= 41
= 42
= 43
= 44
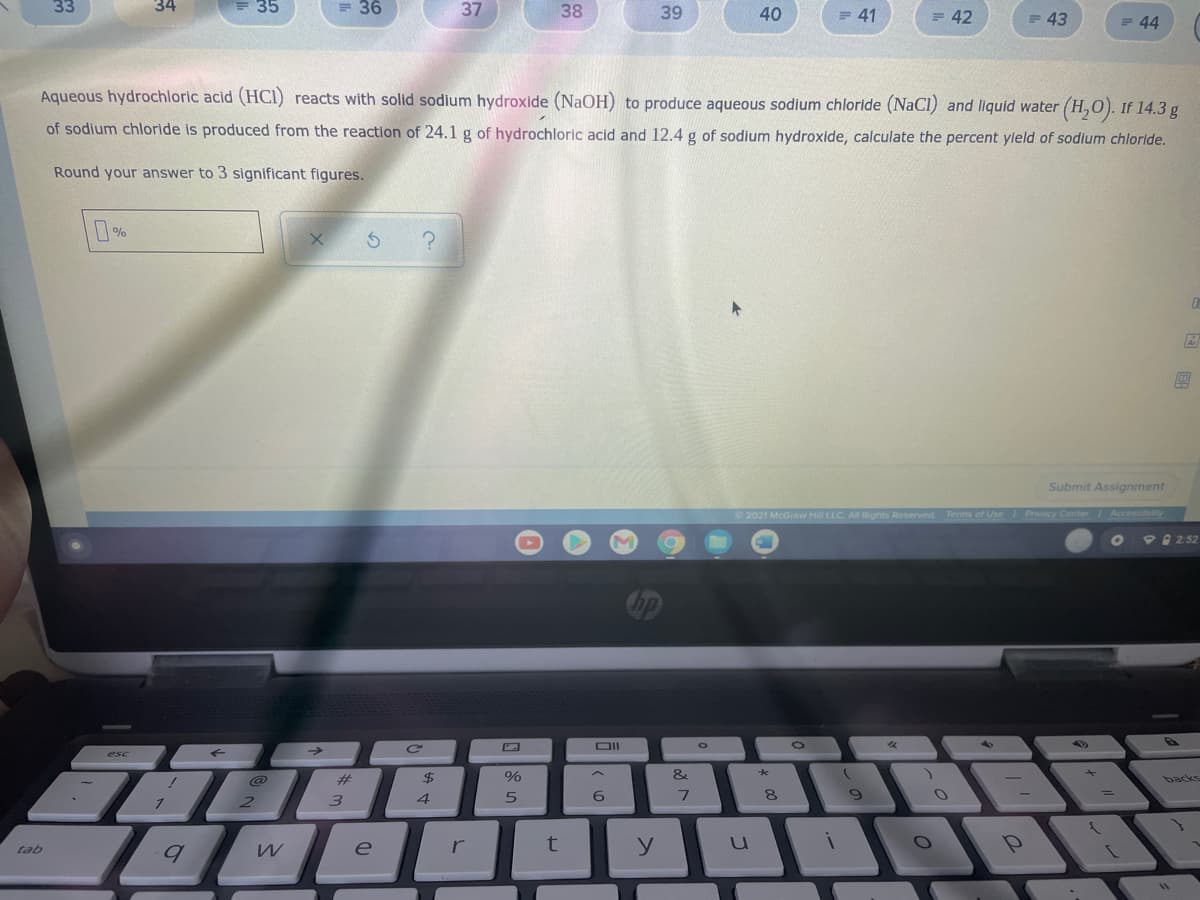
Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H,0). If 14.3 g
of sodium chloride is produced from the reaction of 24.1g of hydrochloric acid and 12.4 g of sodium hydroxide, calculate the percent yleld of sodium chloride.
Round your answer to 3 significant figures.
%
Submit Assignment
2021 McGraw Hill LLC. AN Rights Reserved. Terms of Use Privacy Center Accessibity
P 2:52
DII
->
#
2$
&
backs
3
4
6.
7.
8.
tab
e
r
y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning