4) A sample consisting of 2.00 mol of an ideal gas initially at P 111.0 kPa and T1 277.0 K is heated reversibly to 356.0 K at constant volume. The molar heat capacity at constant volume of the gas is Cy : (5/2) R. Calculate the final pressure, the change in internal energy AU of the gas, and the heat q and work w exchanged between the gas and its surroundings for the process.
4) A sample consisting of 2.00 mol of an ideal gas initially at P 111.0 kPa and T1 277.0 K is heated reversibly to 356.0 K at constant volume. The molar heat capacity at constant volume of the gas is Cy : (5/2) R. Calculate the final pressure, the change in internal energy AU of the gas, and the heat q and work w exchanged between the gas and its surroundings for the process.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.92E
Related questions
Concept explainers
Question
please explain me in detail!! thank you
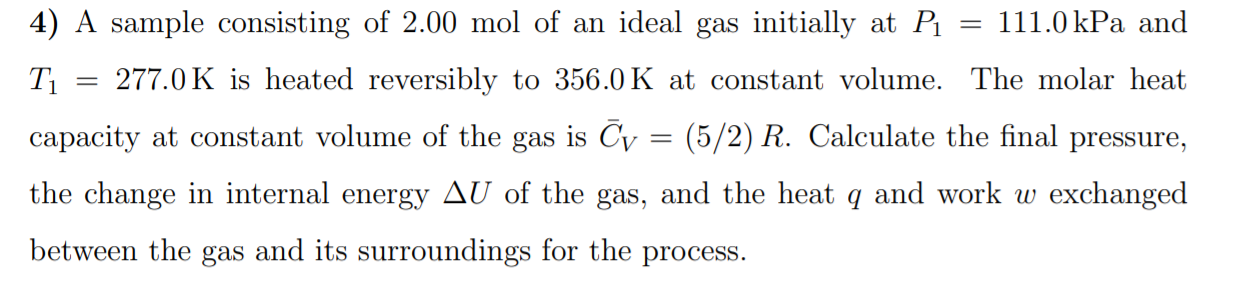
Transcribed Image Text:4) A sample consisting of 2.00 mol of an ideal gas initially at P
111.0 kPa and
T1
277.0 K is heated reversibly to 356.0 K at constant volume. The molar heat
capacity at constant volume of the gas is Cy :
(5/2) R. Calculate the final pressure,
the change in internal energy AU of the gas, and the heat q and work w exchanged
between the gas and its surroundings for the process.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,