4 darts strike near the center of the board. Whoever threw the darts is: * Precise Neither accurate nor precise Accurate Accurate and precise O O O O
4 darts strike near the center of the board. Whoever threw the darts is: * Precise Neither accurate nor precise Accurate Accurate and precise O O O O
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.116PAE
Related questions
Question
URGENT
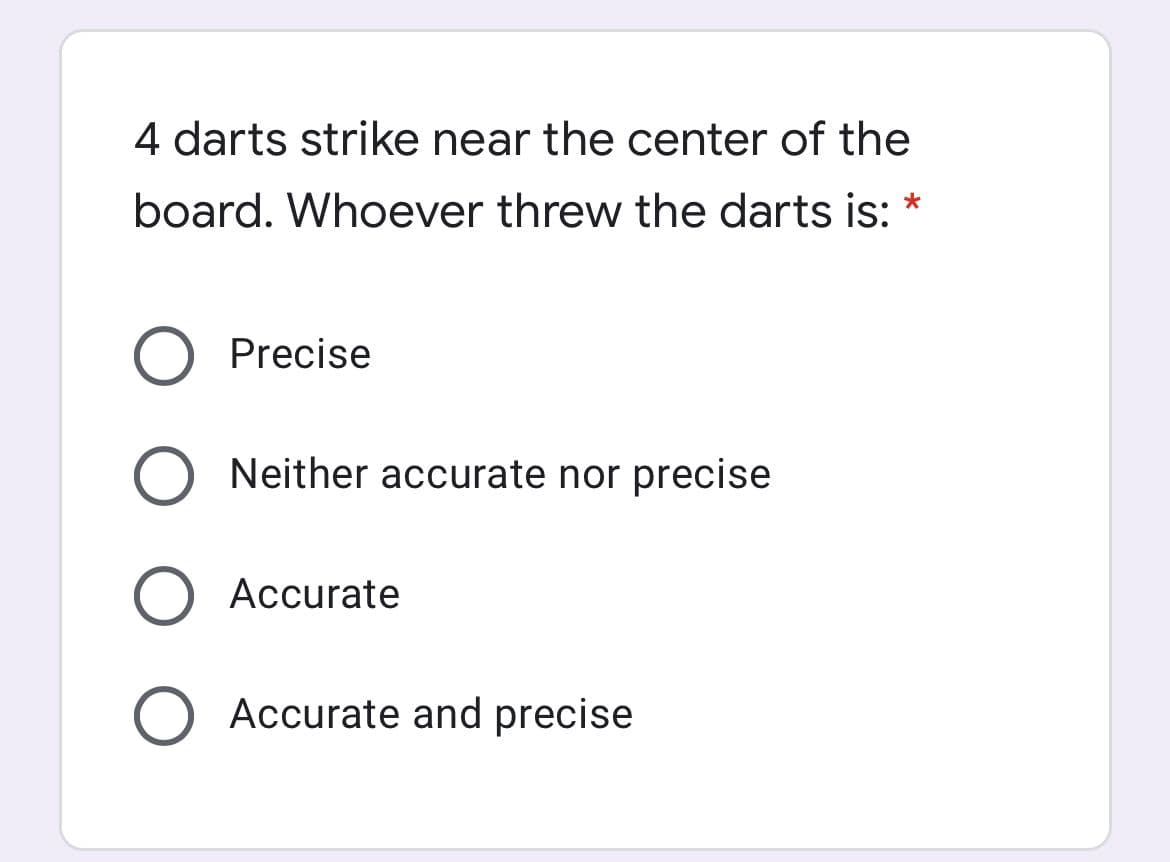
Transcribed Image Text:4 darts strike near the center of the
board. Whoever threw the darts is: *
Precise
Neither accurate nor precise
Accurate
Accurate and precise
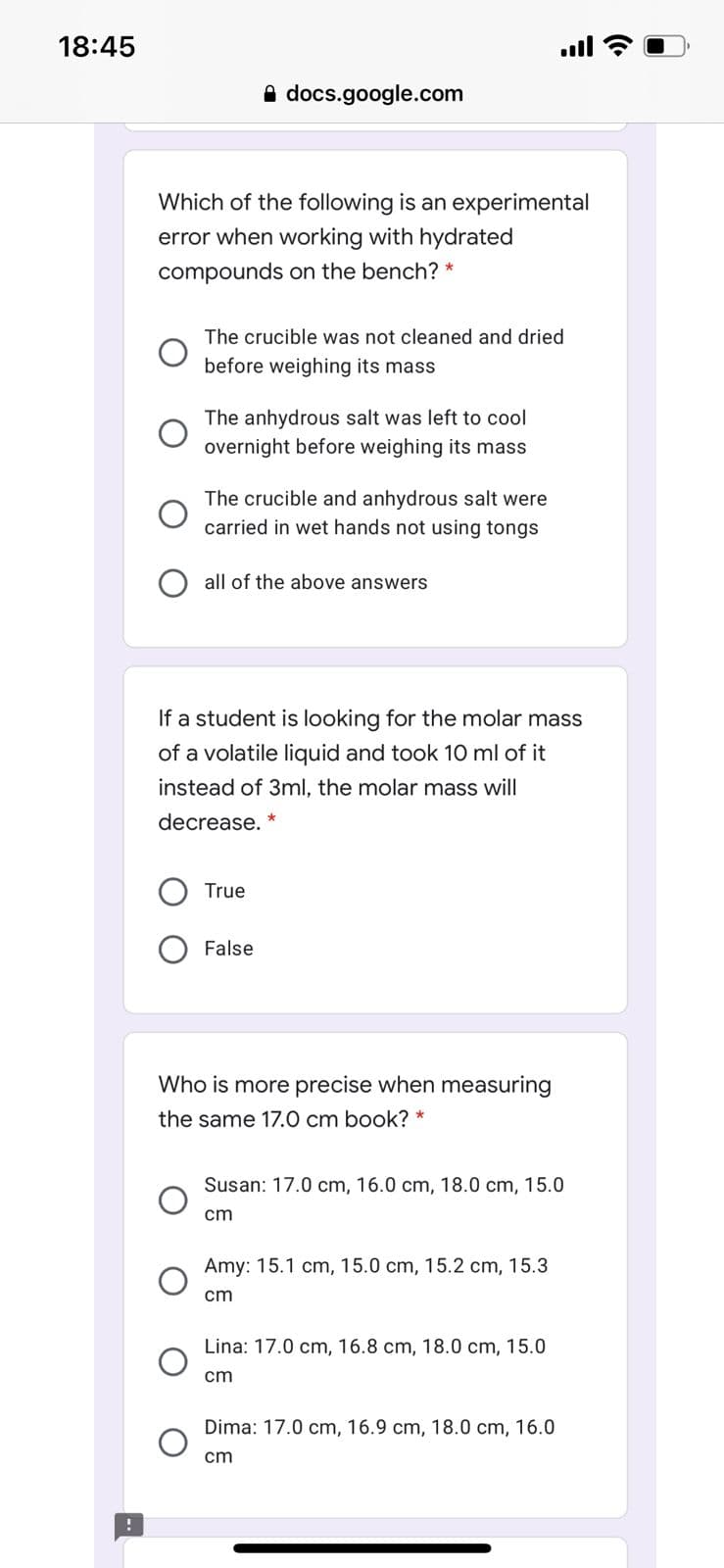
Transcribed Image Text:18:45
ull
A docs.google.com
Which of the following is an experimental
error when working with hydrated
compounds on the bench? *
The crucible was not cleaned and dried
before weighing its mass
The anhydrous salt was left to cool
overnight before weighing its mass
The crucible and anhydrous salt were
carried in wet hands not using tongs
all of the above answers
If a student is looking for the molar mass
of a volatile liquid and took 10 ml of it
instead of 3ml, the molar mass will
decrease. *
True
False
Who is more precise when measuring
the same 17.0 cm book? *
Susan: 17.0 cm, 16.0 cm, 18.0 cm, 15.0
cm
Amy: 15.1 cm, 15.0 cm, 15.2 cm, 15.3
cm
Lina: 17.0 cm, 16.8 cm, 18.0 cm, 15.0
cm
Dima: 17.0 cm, 16.9 cm, 18.0 cm, 16.0
cm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,