Which of the following statements islare true about alkyl halides?" They are non-polar molecules. They are highly soluble in water. Dipole-dipole interaction is present between alkyl halide molecules. All alkyl halides are capable of hydrogen bonding. Alcohols have higher boiling point in comparison to other hydrocarbons of comparable molecular masses because of the presence of hydrogen bonding ion-dipole interaction induced dipole-dipole interaction dipole-dipole interaction Oxidation of isopropyl alcohol (2-Propanol) will produce Methyl ethanoate Propanone Propanal Propanoic acid O O
Which of the following statements islare true about alkyl halides?" They are non-polar molecules. They are highly soluble in water. Dipole-dipole interaction is present between alkyl halide molecules. All alkyl halides are capable of hydrogen bonding. Alcohols have higher boiling point in comparison to other hydrocarbons of comparable molecular masses because of the presence of hydrogen bonding ion-dipole interaction induced dipole-dipole interaction dipole-dipole interaction Oxidation of isopropyl alcohol (2-Propanol) will produce Methyl ethanoate Propanone Propanal Propanoic acid O O
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter23: Addition To A Carbonyl
Section: Chapter Questions
Problem 20E
Related questions
Question
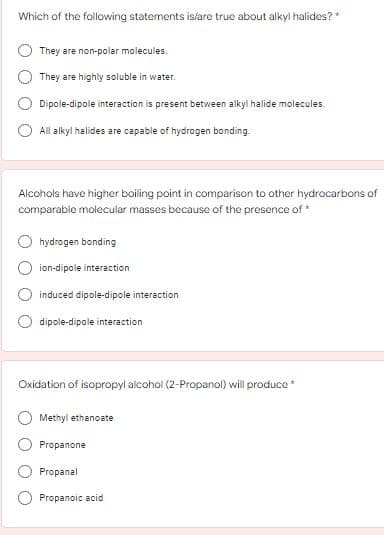
Transcribed Image Text:Which of the following statements islare true about alkyl halides? *
They are non-polar molecules.
They are highly soluble in water.
Dipole-dipole interaction is present between alkyl halide molecules.
All alkyl halides are capable of hydrogen bonding.
Alcohols have higher boiling point in comparison to other hydrocarbons of
comparable molecular masses because of the presence of *
hydrogen bonding
ion-dipole interaction
induced dipole-dipole interaction
dipole-dipole interaction
Oxidation of isopropyl alcohol (2-Propanol) will produce *
Methyl ethanoate
Propanone
Propanal
Propanoic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning