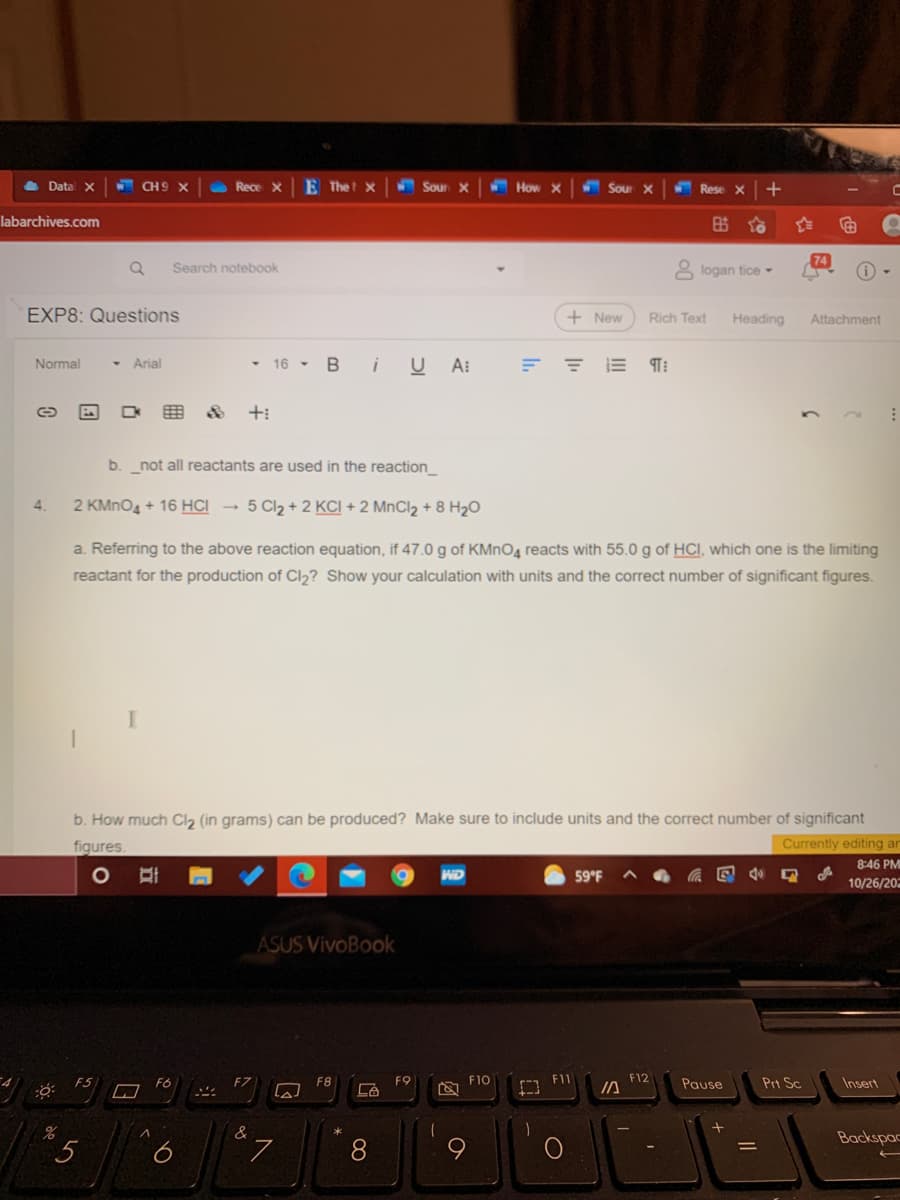
4. 2 KMNO4 + 16 HCI - 5 Cl2 + 2 KCI + 2 MnCl2 + 8 H2O a. Referring to the above reaction equation, if 47.0 g of KMNO4 reacts with 55.0 g of HCI, which one is the limiting reactant for the production of Cl2? Show your calculation with units and the correct number of significant figures. b. How much Cl2 (in grams) can be produced? Make sure to include units and the correct number of significant figures Currently editing
4. 2 KMNO4 + 16 HCI - 5 Cl2 + 2 KCI + 2 MnCl2 + 8 H2O a. Referring to the above reaction equation, if 47.0 g of KMNO4 reacts with 55.0 g of HCI, which one is the limiting reactant for the production of Cl2? Show your calculation with units and the correct number of significant figures. b. How much Cl2 (in grams) can be produced? Make sure to include units and the correct number of significant figures Currently editing
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 95AP: 95. Many metal ions form insoluble sulfide compounds when a solution of the metal ion is treated...
Related questions
Question
Transcribed Image Text:A Data X
A CH9 X
O Rece X
E Thet x
Sour X
How X
Sour x
Rese X
labarchives.com
Search notebook
2 logan tice -
EXP8: Questions
+ New
Rich Text
Heading
Attachment
Normal
- 16 - B iU A:
Arial
& +:
b. _not all reactants are used in the reaction_
4.
2 KMNO4 + 16 HCI → 5 Cl2 + 2 KCI + 2 MNCI2 + 8 H2O
a. Referring to the above reaction equation, if 47.0 g of KMNO4 reacts with 55.0 g of HCI, which one is the limiting
reactant for the production of Cl2? Show your calculation with units and the correct number of significant figures.
b. How much Cl2 (in grams) can be produced? Make sure to include units and the correct number of significant
Currently editing ar
figures.
8:46 PM
WD
59°F
10/26/202
ASUS VivoBook
F10
F11
F12
Pause
Prt Sc
Insert
&
Backspac
8
立
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

