a) Write a balanced chemical reaction for this process , including phases of matter. b)If you started with 1.00 g of ammonium carbonate, how much of each product could be produced? c) What is the limiting reactant? How much ammonium carbonate is needed to produce at least 1.00 g of each product? d)Using the reactant mass from part , what is the percent yield of the reaction if 0.123 g of
a) Write a balanced chemical reaction for this process , including phases of matter. b)If you started with 1.00 g of ammonium carbonate, how much of each product could be produced? c) What is the limiting reactant? How much ammonium carbonate is needed to produce at least 1.00 g of each product? d)Using the reactant mass from part , what is the percent yield of the reaction if 0.123 g of
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 70A
Related questions
Question
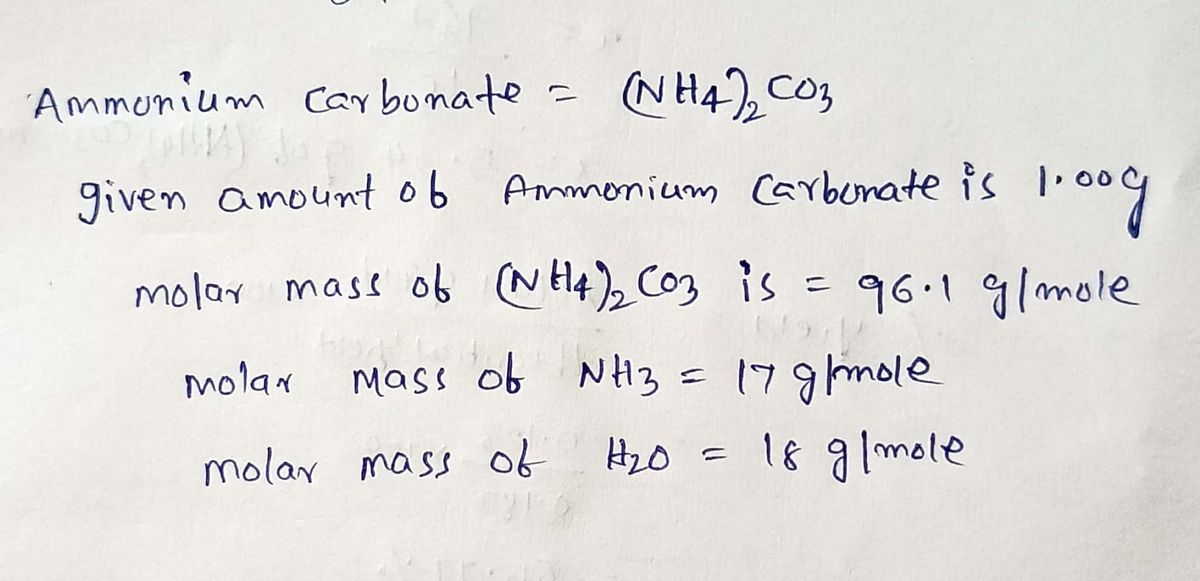
Food: In certain Eastern European desserts it is preferred to use ammonium carbonate as the leavening agent since the decomposition products completely leave the finished product. The leavening reaction: ammonium carbonate decomposes into ammonia, carbon dioxide and water.
a) Write a balanced chemical reaction for this process , including phases of matter.
b)If you started with 1.00 g of ammonium carbonate, how much of each product could be produced?
c) What is the limiting reactant? How much ammonium carbonate is needed to produce at least 1.00 g of each product?
d)Using the reactant mass from part , what is the percent yield of the reaction if 0.123 g of water is produced?
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning