4. Complete the table for the carbon atom s listed from the Lewis Structure below. Determine the electron pair geom etry (EPG), the molecular geom etry (MG), the orbital hybridization and the bond angles around each. Н H-P C,=C;-C,=N-H Н carbon EPG MG Irybridization bond angles C2 C4
4. Complete the table for the carbon atom s listed from the Lewis Structure below. Determine the electron pair geom etry (EPG), the molecular geom etry (MG), the orbital hybridization and the bond angles around each. Н H-P C,=C;-C,=N-H Н carbon EPG MG Irybridization bond angles C2 C4
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter1: Covalent Bonding And Shapes Of Molecules
Section: Chapter Questions
Problem 1.71AP: In Chapter 6, we study a group of organic cations called carbocations. Following is the structure of...
Related questions
Question
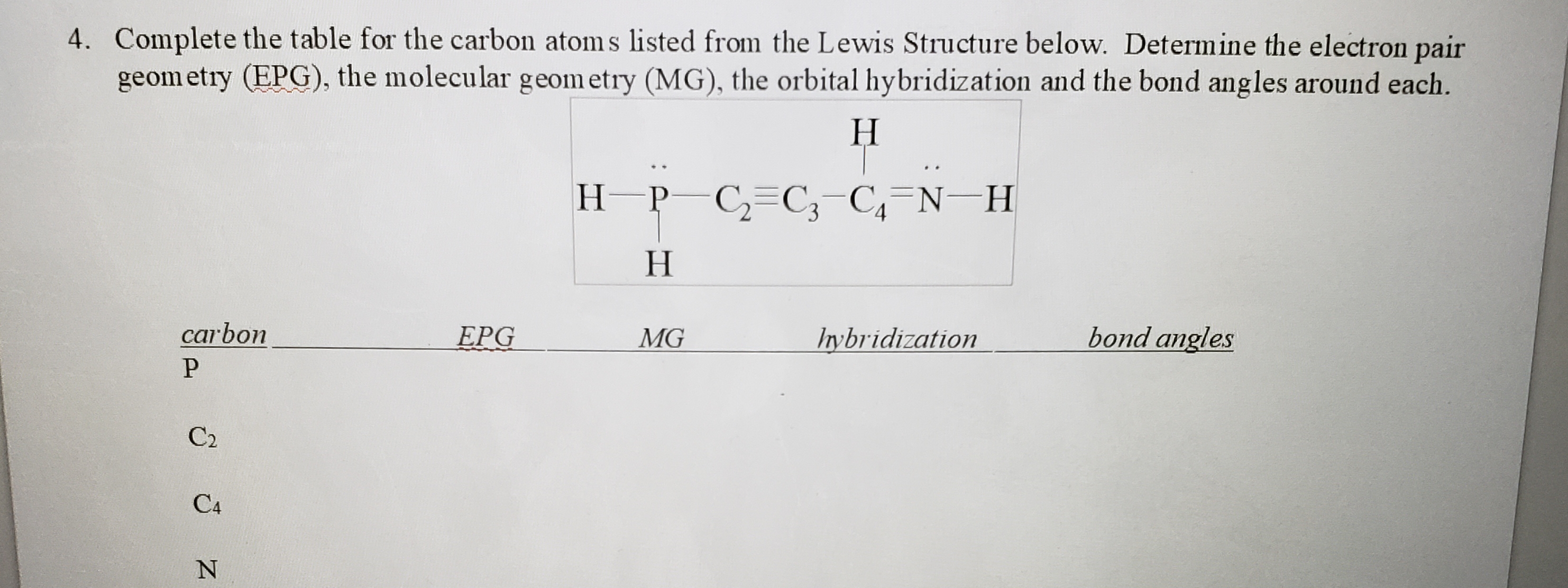
Transcribed Image Text:4. Complete the table for the carbon atom s listed from the Lewis Structure below. Determine the electron pair
geom etry (EPG), the molecular geom etry (MG), the orbital hybridization and the bond angles around each.
Н
H-P C,=C;-C,=N-H
Н
carbon
EPG
MG
Irybridization
bond angles
C2
C4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning