Complete the following table: Lewis structure atoms: bonds between orbitals making each total bond order hybridization atoms: type E valence bond: between two atoms |Н: 1s C and H: o sp?(C) + 1s(H) C and H: 1 C: sp? Cl: sp3 0: sp? :0: || C and Cl: o sp?(C) + sp°(CI) C and Cl: 1 = sp?(C) + sp²(0) = p(C) + p(0) C and O: o C and O: 2 CI C and O: t C: C and C: C and C: C and C: C: H-C=C-H H: C and H: C and C: H: C and H: N: N and I: N and I: N= I: N and I: C: C and Cl: C and Cl: :Cl Cl: C and Cl: C and Cl: Cl: C and Cl: -c=cl: C and Cl: :Z: :U:
Complete the following table: Lewis structure atoms: bonds between orbitals making each total bond order hybridization atoms: type E valence bond: between two atoms |Н: 1s C and H: o sp?(C) + 1s(H) C and H: 1 C: sp? Cl: sp3 0: sp? :0: || C and Cl: o sp?(C) + sp°(CI) C and Cl: 1 = sp?(C) + sp²(0) = p(C) + p(0) C and O: o C and O: 2 CI C and O: t C: C and C: C and C: C and C: C: H-C=C-H H: C and H: C and C: H: C and H: N: N and I: N and I: N= I: N and I: C: C and Cl: C and Cl: :Cl Cl: C and Cl: C and Cl: Cl: C and Cl: -c=cl: C and Cl: :Z: :U:
Chapter3: Mechanisms
Section: Chapter Questions
Problem 105EQ
Related questions
Question
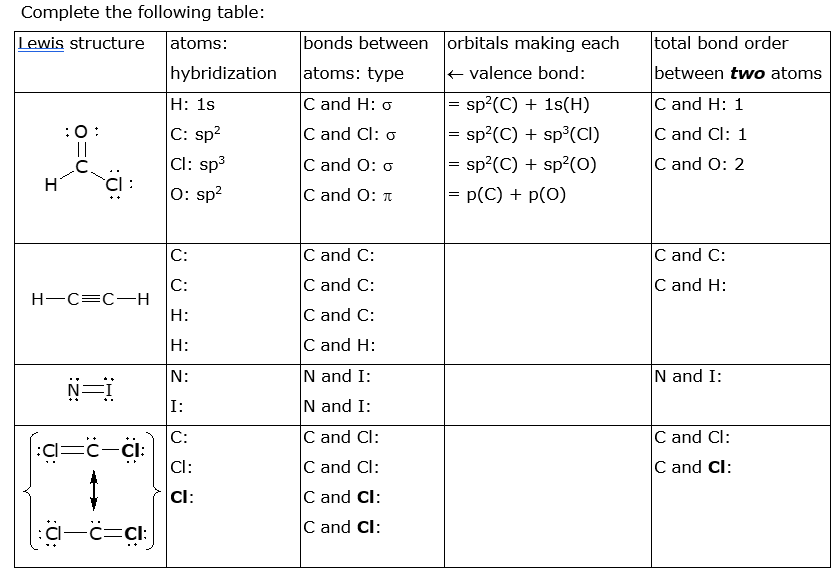
Transcribed Image Text:Complete the following table:
Lewis structure
atoms:
bonds between orbitals making each
total bond order
hybridization
atoms: type
E valence bond:
between two atoms
|Н: 1s
C and H: o
sp?(C) + 1s(H)
C and H: 1
C: sp?
Cl: sp3
0: sp?
:0:
||
C and Cl: o
sp?(C) + sp°(CI)
C and Cl: 1
= sp?(C) + sp²(0)
= p(C) + p(0)
C and O: o
C and O: 2
CI
C and O: t
C:
C and C:
C and C:
C and C:
C:
H-C=C-H
H:
C and H:
C and C:
H:
C and H:
N:
N and I:
N and I:
N=
I:
N and I:
C:
C and Cl:
C and Cl:
:Cl
Cl:
C and Cl:
C and Cl:
Cl:
C and Cl:
-c=cl:
C and Cl:
:Z:
:U:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning