4. Consider the cell below. Draw the cell (using two compartments separated by a porous plate) then answer the questions pertaining to the cell. Al | Al(NO3)3 1.0M || CrCl3 1.0M; CrCl21.0M | Pt a) What will be the spontaneous reaction? b) What will be the cell voltage? c) Show, using an arrow, the direction of flow of the elections through the wire. d) Label the anode and the cathode. e) What is the sign of the anode? f) Show, using arrows and formulas, the direction of flow of ions through the porous plate.
4. Consider the cell below. Draw the cell (using two compartments separated by a porous plate) then answer the questions pertaining to the cell. Al | Al(NO3)3 1.0M || CrCl3 1.0M; CrCl21.0M | Pt a) What will be the spontaneous reaction? b) What will be the cell voltage? c) Show, using an arrow, the direction of flow of the elections through the wire. d) Label the anode and the cathode. e) What is the sign of the anode? f) Show, using arrows and formulas, the direction of flow of ions through the porous plate.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 105QAP: Consider a voltaic cell in which the following reaction occurs. Zn(s)+Sn2+(aq)Zn2+(aq)+Sn(s) (a)...
Related questions
Question

Transcribed Image Text:4. Consider the cell below. Draw the cell (using two compartments separated by a
porous plate) then answer the questions pertaining to the cell.
Al | Al(NO3)3 1.0M || CrCl3 1.0M; CrCl21.0M | Pt
a) What will be the spontaneous reaction?
b) What will be the cell voltage?
c) Show, using an arrow, the direction of flow of the elections through the wire.
d) Label the anode and the cathode.
e) What is the sign of the anode?
f) Show, using arrows and formulas, the direction of flow of ions through the
porous plate.
Expert Solution
Step 1
Since your question has multiple sub-parts, we will solve first three sub-part for you. If you want remaining sub-parts to be solved, then please resubmit the whole question and specify those sub-parts which you want us to solve.
Step 2
For the reaction to be spontaneous, its EMF should be positive. The reactions taking place will be as follows:
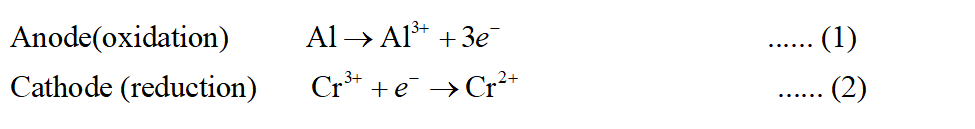
Multiplying equation (2) by 3 so as to cancel the electrons.
The overall spontaneous reaction taking place will be as follows :
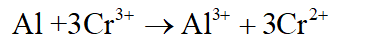
Step by step
Solved in 4 steps with 5 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning