4. Consider the following reaction: I2O5 + CO(g) CO29) + I2(9) a. 85 grams of iodine (V) oxide, I2O5, reacts with carbon monoxide, CO. Determine the mass of iodine I2 that could be produced. b. If in the above situation, only 0.150 moles, of iodine (12) was produced, what is the amount of iodine? c. What is the percent yield of iodine?
4. Consider the following reaction: I2O5 + CO(g) CO29) + I2(9) a. 85 grams of iodine (V) oxide, I2O5, reacts with carbon monoxide, CO. Determine the mass of iodine I2 that could be produced. b. If in the above situation, only 0.150 moles, of iodine (12) was produced, what is the amount of iodine? c. What is the percent yield of iodine?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.117PAE: 3.117 For the oxides of iron, FeO, Fe2O3, and Fe3O4, describe how you would determine which has the...
Related questions
Question
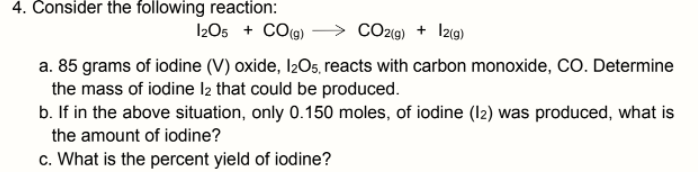
Transcribed Image Text:4. Consider the following reaction:
I2O5 + CO(g)
CO29) + I2(9)
a. 85 grams of iodine (V) oxide, I2O5, reacts with carbon monoxide, CO. Determine
the mass of iodine I2 that could be produced.
b. If in the above situation, only 0.150 moles, of iodine (12) was produced, what is
the amount of iodine?
c. What is the percent yield of iodine?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning