4. If you dissolve lead(II) nitrate and potassium iodide in water they will react to form lead(II) iodide and potassium nitrate. Pb(NO3)2(aq) + 2 Kl(aq) >Pbl2(s) + 2 KNO3(aq) Calculate the grams of lead(II) iodide that can be produced from 85.00 grams of potassium iodide.
4. If you dissolve lead(II) nitrate and potassium iodide in water they will react to form lead(II) iodide and potassium nitrate. Pb(NO3)2(aq) + 2 Kl(aq) >Pbl2(s) + 2 KNO3(aq) Calculate the grams of lead(II) iodide that can be produced from 85.00 grams of potassium iodide.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 83GQ
Related questions
Question
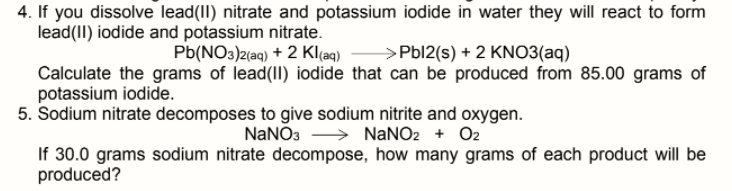
Transcribed Image Text:4. If you dissolve lead(II) nitrate and potassium iodide in water they will react to form
lead(II) iodide and potassium nitrate.
Pb(NO3)2(aq) + 2 Kl(aq)
>Pbl2(s) + 2 KNO3(aq)
Calculate the grams of lead(II) iodide that can be produced from 85.00 grams of
potassium iodide.
5. Sodium nitrate decomposes to give sodium nitrite and oxygen.
NaNO3 > NaNO2 + O2
If 30.0 grams sodium nitrate decompose, how many grams of each product will be
produced?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning