4. Which is more efficient method of separating solids from liquids by filtration or by decantation? Why? 5. Write the balanced chemical equation involved in: a) Precipitation: FeCl3(@a + NaOH(a) (e) + (aq) A (agw b) Evaporation: 6. What is the chemical composition of the following, a. Eiltrate b. Residue -
4. Which is more efficient method of separating solids from liquids by filtration or by decantation? Why? 5. Write the balanced chemical equation involved in: a) Precipitation: FeCl3(@a + NaOH(a) (e) + (aq) A (agw b) Evaporation: 6. What is the chemical composition of the following, a. Eiltrate b. Residue -
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.13QAP
Related questions
Question
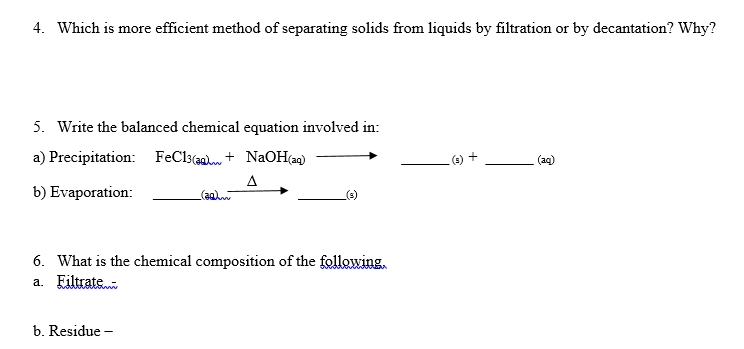
Transcribed Image Text:4. Which is more efficient method of separating solids from liquids by filtration or by decantation? Why?
5. Write the balanced chemical equation involved in:
a) Precipitation: FeCl3(@a + NaOH(a)
(e) +
(aq)
A
(agw
b) Evaporation:
6. What is the chemical composition of the following,
a. Eiltrate
b. Residue -
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning