Trial 1 Trial 2 1-Mass of empty evaporating dish 55.312 g 55.315 g 56.619 g 56.018 g 56.448 g 2- Mass of evaporating dish + MgSO4.nH2O 3-Mass of evaporating containing the salt after heating 4-Mass of MgS04 (line3 - line1) 55.889 g 5- Moles of MgSO4 6- Mass of nH20 (line2- line3) 7- Moles of H2O 8-n= Mole ratio: moles of H2O/Moles of MgSO4 (line7/ line5) Average value of n ( as a decimal): Rounded average value of n: Formula of Magnesium sulfate hydrate:
Trial 1 Trial 2 1-Mass of empty evaporating dish 55.312 g 55.315 g 56.619 g 56.018 g 56.448 g 2- Mass of evaporating dish + MgSO4.nH2O 3-Mass of evaporating containing the salt after heating 4-Mass of MgS04 (line3 - line1) 55.889 g 5- Moles of MgSO4 6- Mass of nH20 (line2- line3) 7- Moles of H2O 8-n= Mole ratio: moles of H2O/Moles of MgSO4 (line7/ line5) Average value of n ( as a decimal): Rounded average value of n: Formula of Magnesium sulfate hydrate:
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.24QAP
Related questions
Question
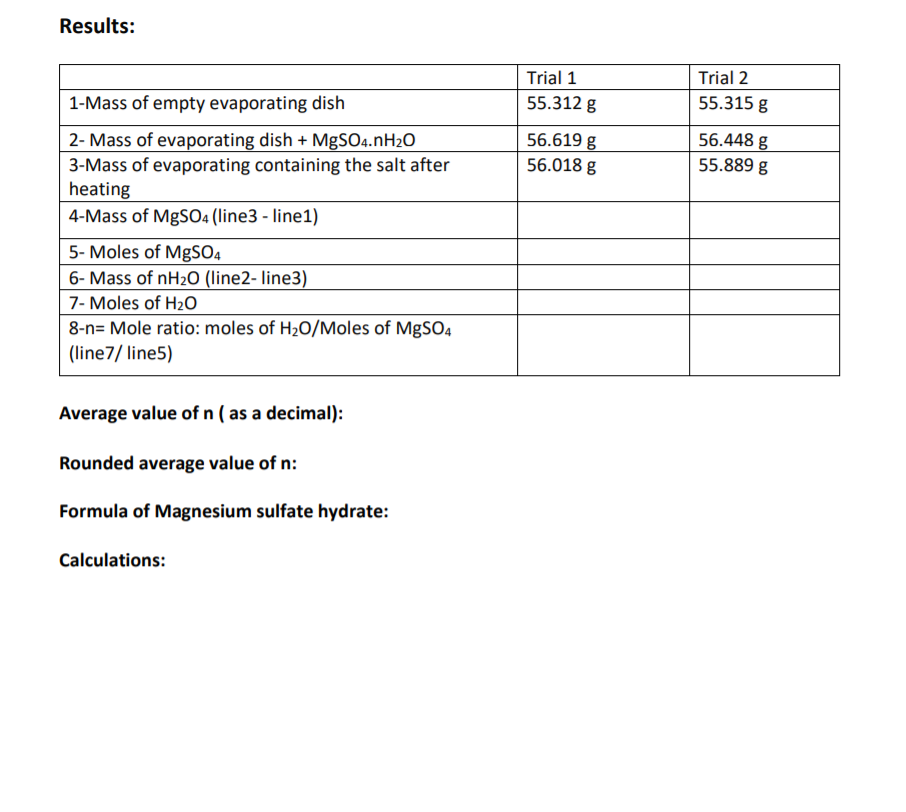
Transcribed Image Text:Results:
Trial 1
Trial 2
1-Mass of empty evaporating dish
55.312 g
55.315 g
2- Mass of evaporating dish + MgSO4.nH2O
3-Mass of evaporating containing the salt after
heating
56.619 g
56.448 g
55.889 g
56.018 g
4-Mass of MgS04 (line3 - line1)
5- Moles of MgSO4
6- Mass of nH20 (line2- line3)
7- Moles of H20
8-n= Mole ratio: moles of H20/Moles of MgSO4
(line7/ line5)
Average value of n ( as a decimal):
Rounded average value of n:
Formula of Magnesium sulfate hydrate:
Calculations:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

