4.90g of Sodium idotae is placed insode a sealed flask and heated to 509.0 k. The compoiund completley decompses according to the blanced chemicla equation below, forming oxygen gas with a preassure of 0.515atm. what is the volume in L of the flask. 2NaIO3->2Nal+ 3O2
4.90g of Sodium idotae is placed insode a sealed flask and heated to 509.0 k. The compoiund completley decompses according to the blanced chemicla equation below, forming oxygen gas with a preassure of 0.515atm. what is the volume in L of the flask. 2NaIO3->2Nal+ 3O2
Stoichiometric ratio depicts proportion/molar ratio among participating reactant species and also product species in known reaction. The ratio gets utilized in obtaining formed species amount (mass/moles).
Given
The mass of sodium iodate is 4.90 g.
The pressure is 0.515 atm.
The temperature is 509.0 K.
The decomposition reaction is shown below.
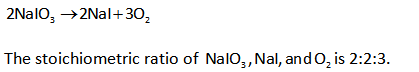
The formula for the calculation of moles of sodium iodate is shown below.
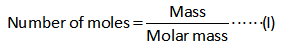
The molar mass of sodium iodate is 197.8924 g/mol.
Substitute the known values in the equation (I).
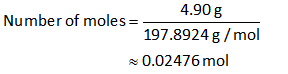
The number of moles of sodium iodate is 0.02476 moles.

Thus the number of moles of O2 can be calculated as shown below,
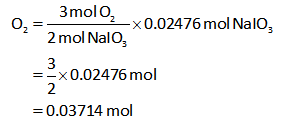
The number of moles of O2 is 0.03714 moles.
The volume can be calculated by using the ideal gas equation shown below.
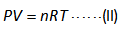
Here,
The pressure is “P”.
The volume is “V”.
The number of moles of the gas is “n”.
The gas constant is “R”.
The temperature is “T”.
Step by step
Solved in 5 steps with 8 images









