436 kJ/mol 431 kJ/mol 567 kJ/mol 413 kJ/mol 348 kJ/mol 293 kJ/mol C=C 614 kJ/mol H-H H-CI C-H C-C C=C 839 kJ/mol 799 kJ/mol H-F C-N C-O C-F N-H 391 kJ/mol 358 kJ/mol 495 kJ/mol N-O 201 kJ/mol 485 kJ/mol C=O 1072 kJ/mol O-H O-0 C=N N=N 463 kJ/mol 328 kJ/mol 615 kJ/mol C-S CI-CI 146 kJ/mol 259 kJ/mol 418 kJ/mol F-F 155 kJ/mol 242 kJ/mol N=N 941 kJ/mol C=N 891 kJ/mol Estimate the enthalpy change (A Hrxn) of the following reaction for 5 moles of Cl2 using the bond energies above. H-H + CI-CI → H-CI + H-CI kJ
436 kJ/mol 431 kJ/mol 567 kJ/mol 413 kJ/mol 348 kJ/mol 293 kJ/mol C=C 614 kJ/mol H-H H-CI C-H C-C C=C 839 kJ/mol 799 kJ/mol H-F C-N C-O C-F N-H 391 kJ/mol 358 kJ/mol 495 kJ/mol N-O 201 kJ/mol 485 kJ/mol C=O 1072 kJ/mol O-H O-0 C=N N=N 463 kJ/mol 328 kJ/mol 615 kJ/mol C-S CI-CI 146 kJ/mol 259 kJ/mol 418 kJ/mol F-F 155 kJ/mol 242 kJ/mol N=N 941 kJ/mol C=N 891 kJ/mol Estimate the enthalpy change (A Hrxn) of the following reaction for 5 moles of Cl2 using the bond energies above. H-H + CI-CI → H-CI + H-CI kJ
Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.SE: Something Extra
Problem 33AP
Related questions
Question
100%
Im confuse on how to solve this exercise
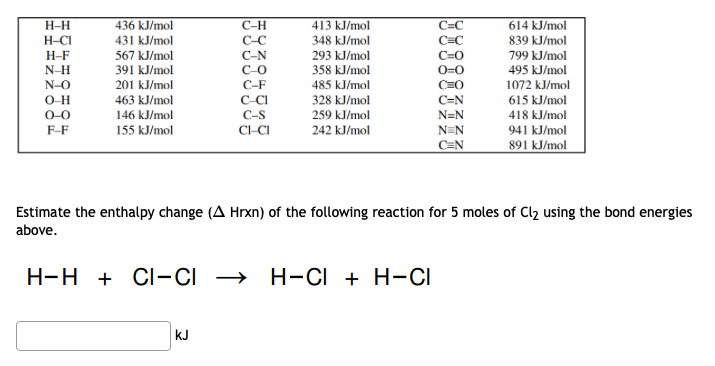
Transcribed Image Text:Н-Н
436 kJ/mol
С-Н
413 kJ/mol
C=C
614 kJ/mol
H-CI
C-C
C-N
C-O
431 kJ/mol
348 kJ/mol
839 kJ/mol
H-F
567 kJ/mol
293 kJ/mol
799 kJ/mol
N-H
391 kJ/mol
358 kJ/mol
495 kJ/mol
N-O
O-H
201 kJ/mol
С-F
485 kJ/mol
1072 kJ/mol
463 kJ/mol
C-CI
328 kJ/mol
615 kJ/mol
418 kJ/mol
941 kJ/mol
891 kJ/mol
O-0
146 kJ/mol
C-S
259 kJ/mol
N=N
F-F
155 kJ/mol
CI-CI
242 kJ/mol
N=N
C=N
Estimate the enthalpy change (A Hrxn) of the following reaction for 5 moles of Cl2 using the bond energies
above.
H-H + CI-CI →
→ H-CI + H-CI
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER