5) each carbon atom. Subane C8H8 (s) is a cubic-shaped hydrocarbon with a carbon atom at each corner of the cube. H ecular Cubane is very unstable. Some researchers have been seriously injured when crystals of the compound exploded while being scooped out of a bottle. Not surprisingly, cubane has been the subject of some research as an explosive. a. According to the VSEPR theory, what should be the shape around each carbon atom? What bond angle is associated with this shape? b. If you assume an ideal cubic shape, what are the actual bond angles around each carbon? c. Explain how your answers to questions 5a and 5b suggest why this molecule is so unstable.
5) each carbon atom. Subane C8H8 (s) is a cubic-shaped hydrocarbon with a carbon atom at each corner of the cube. H ecular Cubane is very unstable. Some researchers have been seriously injured when crystals of the compound exploded while being scooped out of a bottle. Not surprisingly, cubane has been the subject of some research as an explosive. a. According to the VSEPR theory, what should be the shape around each carbon atom? What bond angle is associated with this shape? b. If you assume an ideal cubic shape, what are the actual bond angles around each carbon? c. Explain how your answers to questions 5a and 5b suggest why this molecule is so unstable.
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 42CR
Related questions
Concept explainers
Bond Parameters
Many factors decide the covalent bonding between atoms. Some of the bond parameters are bond angle, bond order, enthalpy, bond length, etc. These parameters decide what kind of bond will form in atoms. Hence it is crucial to understand these parameters in detail and understand how changing these parameters affects the kind of bonding or various characteristics.
Bond Dissociation Energy
The tendency of an atom to attract an electron is known as its electronegativity.
Question
How can we answer question 5 ?
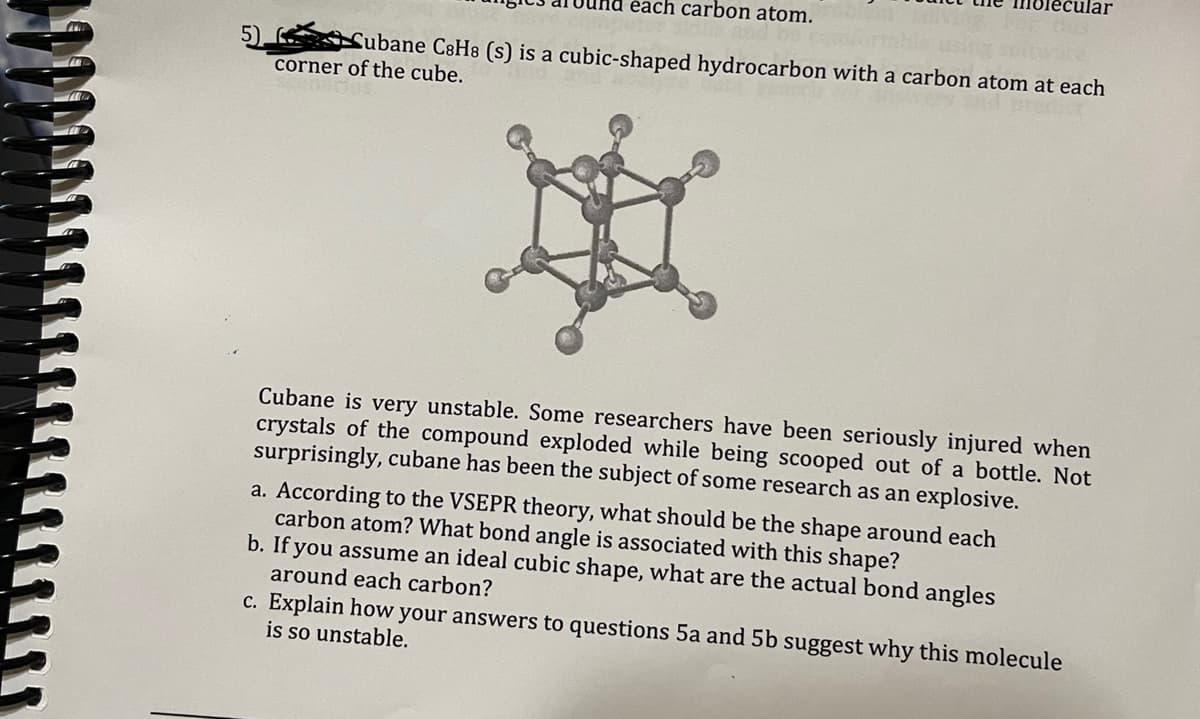
Transcribed Image Text:5)
each carbon atom.
Cubane C8H8 (s) is a cubic-shaped hydrocarbon with a carbon atom at each
corner of the cube.
H
cular
Cubane is very unstable. Some researchers have been seriously injured when
crystals of the compound exploded while being scooped out of a bottle. Not
surprisingly, cubane has been the subject of some research as an explosive.
a. According to the VSEPR theory, what should be the shape around each
carbon atom? What bond angle is associated with this shape?
b. If you assume an ideal cubic shape, what are the actual bond angles
around each carbon?
c. Explain how your answers to questions 5a and 5b suggest why this molecule
is so unstable.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning