5. (a) (b) (c) Studying the Temperature Dependence of the Rate Constant (k). The following reaction is studied at 3 different temperatures. Below you I will find the rate constant for a reaction determined at these temperatures. Use this data to answer the following questions k (min) 0.0409 min 0.0818 min -1 0.157 min -1 T (Celsius) 25.0 °C 35.0 °C 45.0 °C Calculate In k and 1/T and provide the values in the table below. In k 1/T (K¹) Plot In k vs. 1/T and attach the graph. It should be linear. A > B Calculate the slope of the graph of In k vs. 1/T. Show your work below. (d) Determine the Activation Energy of this reaction using the slope above. Show work below.
5. (a) (b) (c) Studying the Temperature Dependence of the Rate Constant (k). The following reaction is studied at 3 different temperatures. Below you I will find the rate constant for a reaction determined at these temperatures. Use this data to answer the following questions k (min) 0.0409 min 0.0818 min -1 0.157 min -1 T (Celsius) 25.0 °C 35.0 °C 45.0 °C Calculate In k and 1/T and provide the values in the table below. In k 1/T (K¹) Plot In k vs. 1/T and attach the graph. It should be linear. A > B Calculate the slope of the graph of In k vs. 1/T. Show your work below. (d) Determine the Activation Energy of this reaction using the slope above. Show work below.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.57PAE
Related questions
Question
100%
A-C pls
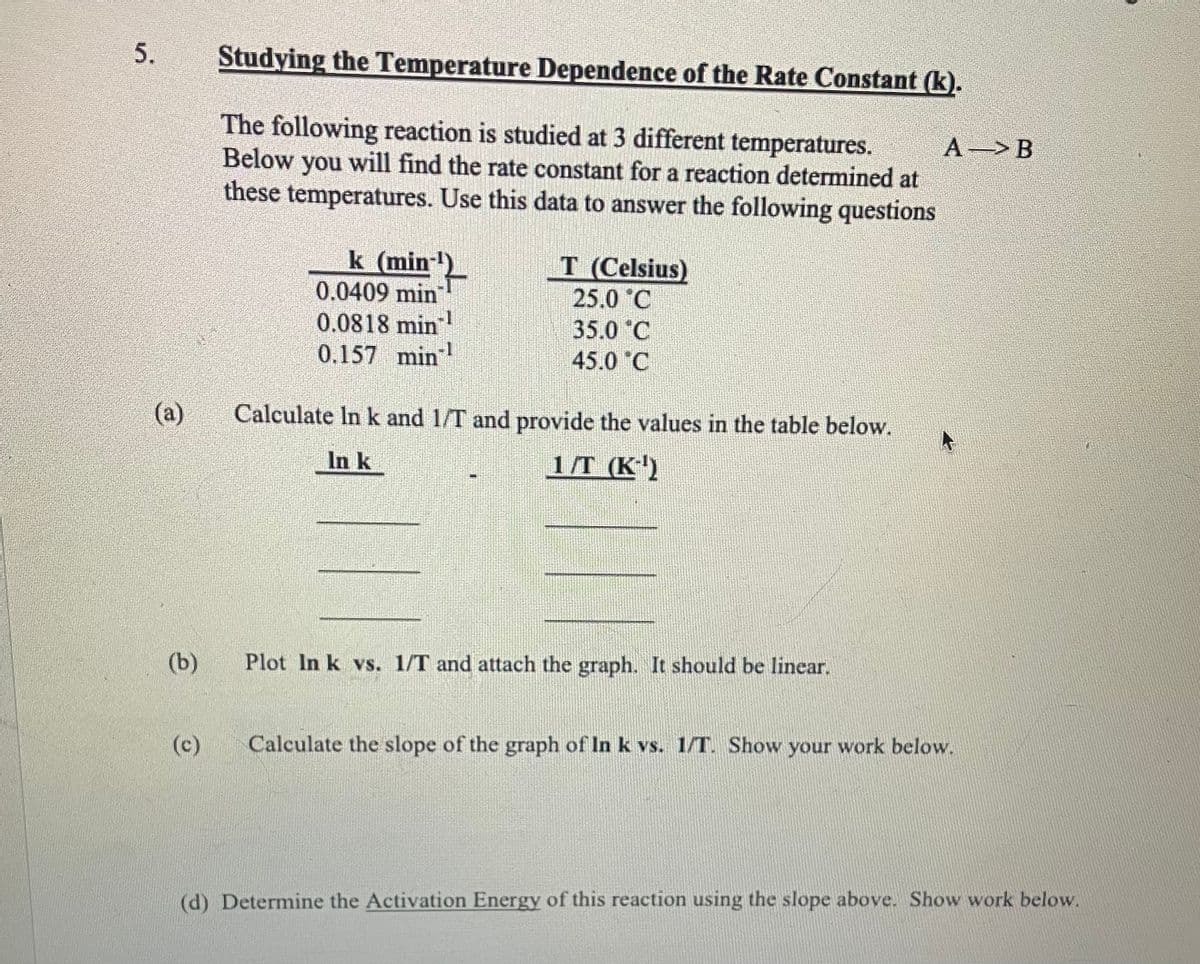
Transcribed Image Text:5.
(a)
(b)
(c)
Studying the Temperature Dependence of the Rate Constant (k).
The following reaction is studied at 3 different temperatures.
Below you will find the rate constant for a reaction determined at
these temperatures. Use this data to answer the following questions
k (min
0.0409 min
0.0818 min
0.157 min -1
T (Celsius)
25.0 C
35.0 °C
45.0 C
Calculate In k and 1/T and provide the values in the table below.
In k
1/T (K-¹)
Plot In k vs. 1/T and attach the graph. It should be linear.
A B
Calculate the slope of the graph of In k vs. 1/T. Show your work below.
(d) Determine the Activation Energy of this reaction using the slope above. Show work below.
Expert Solution
Step 1
The Arrhenius equation is
The Arrhenius equation is in the straight line form with
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning