Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated, for several elements; for those that are nonmetals, only atomic radii are indicated. Atomic Radius Crystal Structure negativity Valence Electro- Element (пт) Ni 0.1246 FCC 1.8 +2 0.071 H 0.046 0.060 FCC 1.9 1.5 +1 Ag Al Co 0.1445 0.1431 FCC +3 +2 +3 +2 +2 +2 0.1253 НСР ВСС 1.8 Cr 0.1249 1.6 Fe 0.1241 0.1387 0.1332 ВСС 1.8 2.2 1.6 Pt FCC Zn HCP Which of these elements would you expect to form the following with nickel: (a) A substitutional solid solution having complete solubility (b) A substitutional solid solution of incomplete solubility (c) An interstitial solid solution
Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated, for several elements; for those that are nonmetals, only atomic radii are indicated. Atomic Radius Crystal Structure negativity Valence Electro- Element (пт) Ni 0.1246 FCC 1.8 +2 0.071 H 0.046 0.060 FCC 1.9 1.5 +1 Ag Al Co 0.1445 0.1431 FCC +3 +2 +3 +2 +2 +2 0.1253 НСР ВСС 1.8 Cr 0.1249 1.6 Fe 0.1241 0.1387 0.1332 ВСС 1.8 2.2 1.6 Pt FCC Zn HCP Which of these elements would you expect to form the following with nickel: (a) A substitutional solid solution having complete solubility (b) A substitutional solid solution of incomplete solubility (c) An interstitial solid solution
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter11: Liquids And Solids
Section: Chapter Questions
Problem 11.24QE: An amorphous solid can sometimes be converted to a crystalline solid by a process called annealing....
Related questions
Question
ımages
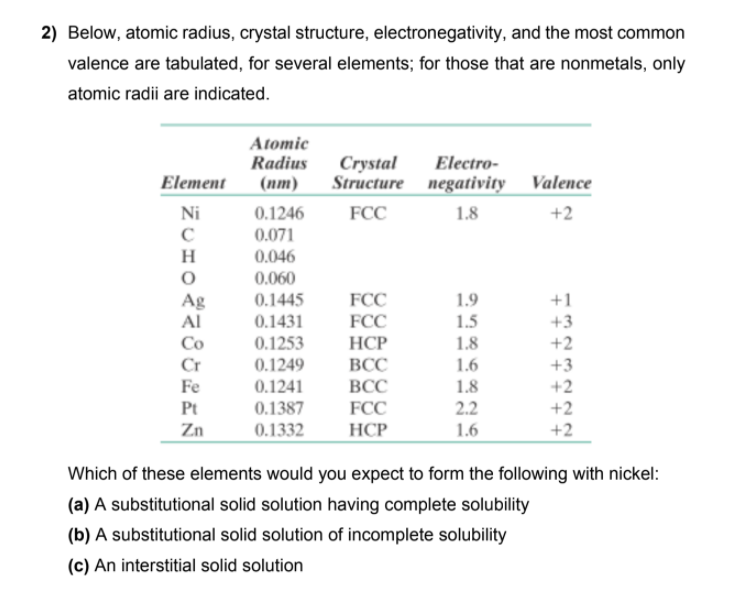
Transcribed Image Text:2) Below, atomic radius, crystal structure, electronegativity, and the most common
valence are tabulated, for several elements; for those that are nonmetals, only
atomic radii are indicated.
Atomic
Radius
(пт)
Crystal
Structure
Electro-
Element
negativity Valence
Ni
1.8
+2
0.1246
0.071
FCC
H
0.046
0.060
Ag
Al
0.1445
0.1431
FCC
1.9
1.5
+1
FCC
HCP
+3
+2
Co
0.1253
1.8
Cr
Fe
0.1249
ВСС
1.6
+3
+2
0.1241
ВСС
1.8
Pt
0.1387
FCC
2.2
+2
+2
Zn
0.1332
HCP
1.6
Which of these elements would you expect to form the following with nickel:
(a) A substitutional solid solution having complete solubility
(b) A substitutional solid solution of incomplete solubility
(c) An interstitial solid solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning