5. Calculate the new volume of a particular gas if 1.23 L of it, initially at a temperature of 32.0 °C is subjected to a drop in temperature of 19.0 degrees Celsius. (1)
5. Calculate the new volume of a particular gas if 1.23 L of it, initially at a temperature of 32.0 °C is subjected to a drop in temperature of 19.0 degrees Celsius. (1)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 91E: Can the speed of a given molecule in a gas double at constant temperature? Explain your answer.
Related questions
Question
Please workout this problem for me, an example of what would be best for me is the screenshot with a paper I dont really understand it typed out. Thank you so much!!!! :) I appreciate anyone who could do it for me.
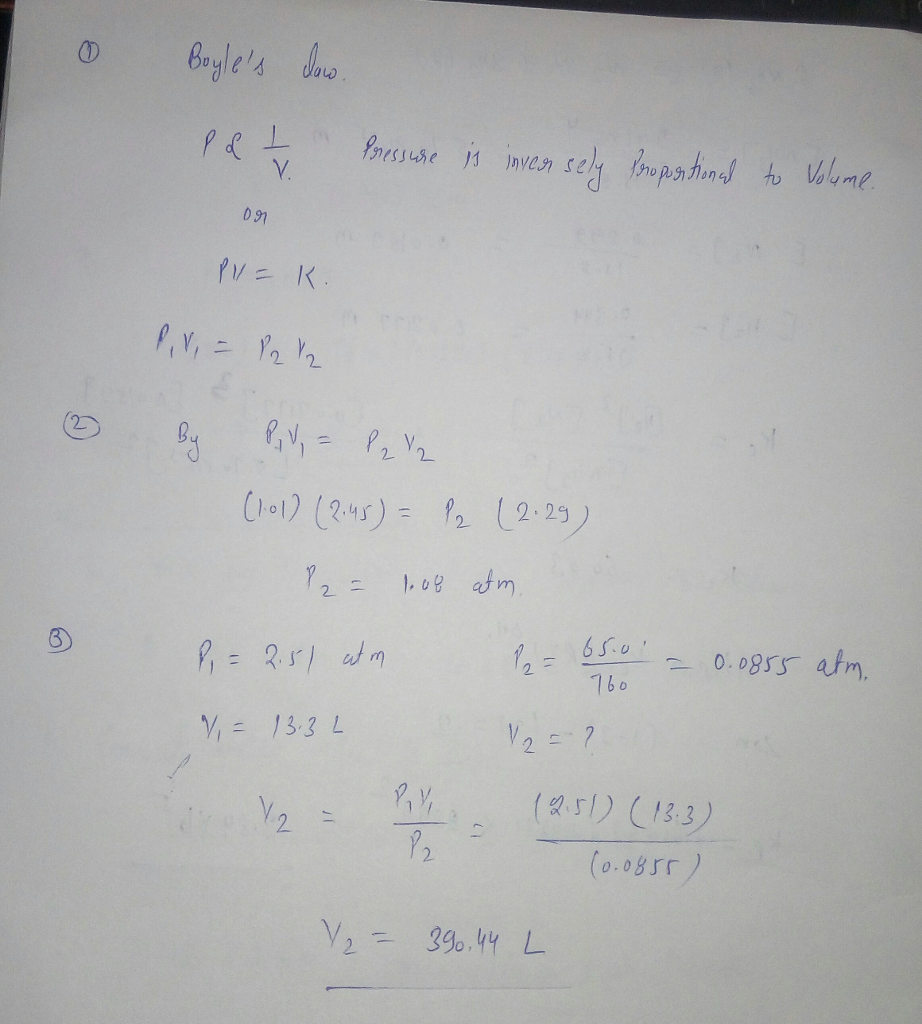
Transcribed Image Text:boyle's dnw.
Prussuse is inven sely Propstinal to Volume.
V.
PV= K.
%3D
(h01) (2.45) = Pz (2:25)
%3D
1, 08 afm
P,= 2.51 at m
65.0'
- 0.0855 atm.
760
V, = 13.3 L
V2 = ?
V2 =
(251) (13.3)
(0.0855)
V2 = 396.44 L

Transcribed Image Text:5. Calculate the new volume of a particular gas if 1.23 L of it, initially at a temperature of 32.0 °C
is subjected to a drop in temperature of 19.0 degrees Celsius. (1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning