5. Draw Lewis structures for each of the following covalent molecules, using two dots to represent an electron pair. Show bonding and non-bonding valence level electrons on all atoms. (i) water (ii) ammonia (iii) methane (iv) carbon dioxide (v) ethylene (vi) acetylene (vii) nitrogen trifluoride (viii) carbon tetrachloride (ix) silicon tetrachloride (x) nitrogen tribromide
5. Draw Lewis structures for each of the following covalent molecules, using two dots to represent an electron pair. Show bonding and non-bonding valence level electrons on all atoms. (i) water (ii) ammonia (iii) methane (iv) carbon dioxide (v) ethylene (vi) acetylene (vii) nitrogen trifluoride (viii) carbon tetrachloride (ix) silicon tetrachloride (x) nitrogen tribromide
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 86AP
Related questions
Question
100%
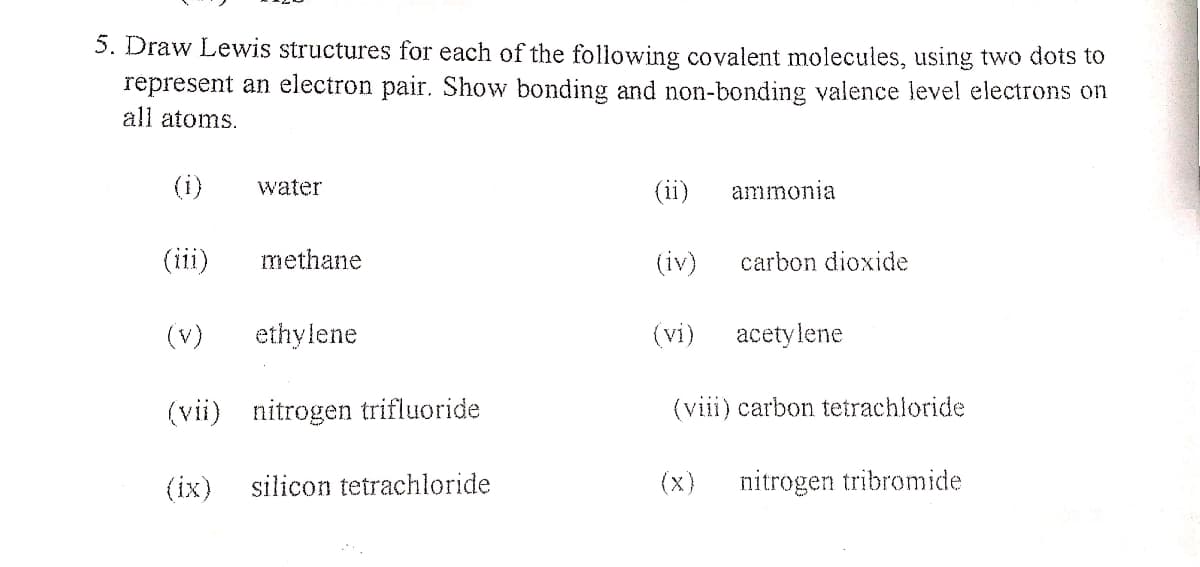
Transcribed Image Text:5. Draw Lewis structures for each of the following covalent molecules, using two dots to
represent an electron pair. Show bonding and non-bonding valence level electrons on
all atoms.
(i)
water
(ii)
ammonia
(iii)
methane
(iv)
carbon dioxide
(v)
ethylene
(vi)
acetylene
(vii) nitrogen trifluoride
(viii) carbon tetrachloride
(ix)
silicon tetrachloride
(x)
nitrogen tribromide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning