5. Here, you can find an example of a paper chromatography experiment. Use the two Chromatograms below the answer the questions that follow: a) (3p) What are the components of unknown sample (X)? b) (12p) Calculate the Rf values for A, B, C in the first chromatogram. d) (10p) When using the 20% Acetone in Water, are the substances strongly attracted to the stationary phase or the mobile Phase? Briefly explain your reasoning. e) (10p) Which substance does show strongest affinity to the stationary phase in the first chromatogram. Briefly explain your reasoning. wwan wwww www 50 80% Acetone in water 20% Acetone in water Finish line 50 45 45 40 40 35 35 30 30 25 20 15 10 10 Starting line A B C D X A B C D X
5. Here, you can find an example of a paper chromatography experiment. Use the two Chromatograms below the answer the questions that follow: a) (3p) What are the components of unknown sample (X)? b) (12p) Calculate the Rf values for A, B, C in the first chromatogram. d) (10p) When using the 20% Acetone in Water, are the substances strongly attracted to the stationary phase or the mobile Phase? Briefly explain your reasoning. e) (10p) Which substance does show strongest affinity to the stationary phase in the first chromatogram. Briefly explain your reasoning. wwan wwww www 50 80% Acetone in water 20% Acetone in water Finish line 50 45 45 40 40 35 35 30 30 25 20 15 10 10 Starting line A B C D X A B C D X
Chapter88: Column Chromatography
Section: Chapter Questions
Problem 4P
Related questions
Question
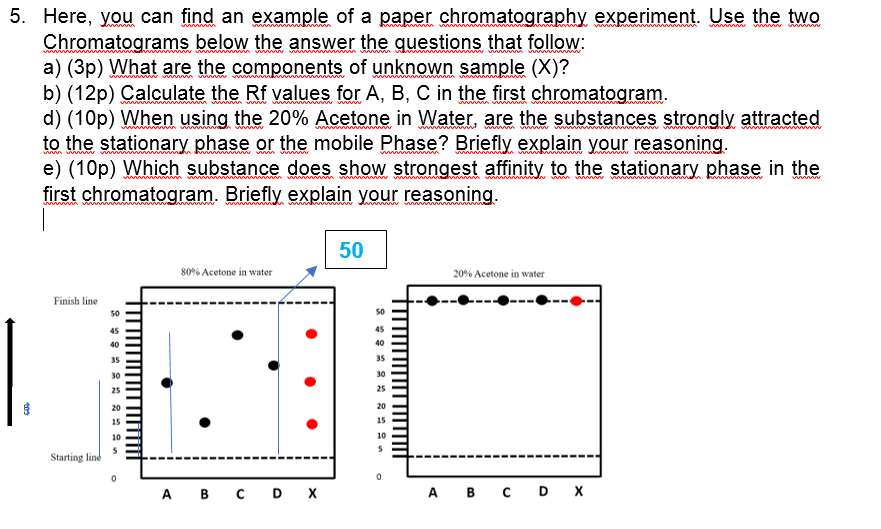
Transcribed Image Text:5. Here, you can find an example of a paper chromatography experiment. Use the two
Chromatograms below the answer the questions that follow:
a) (3p) What are the components of unknown sample (X)?
b) (12p) Calculate the Rf values for A, B, C in the first chromatogram.
d) (10p) When using the 20% Acetone in Water, are the substances strongly attracted
to the stationary phase or the mobile Phase? Briefly explain your reasoning.
e) (10p) Which substance does show strongest affinity to the stationary phase in the
first chromatogram. Briefly explain your reasoning.
wwan
www
50
80% Acetone in water
20% Acetone in water
Finish line
50
45
45
40
40
35
35
30
30
25
20
15
10
10
Starting line
A B C D X
A B C D X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning