5. In the Bohr model for hydrogen, the radius of the nth orbit can be shown to be ³ times the radius of the first Bohr orbit r,-0.05 nm (see Example 10.3). Similarly, the energy of an electron in the nth orbit is times its energy when in the n 1 orbit. What is the circumference of the n = 100 orbit? (This is the distance the electron has traveled after having revolved around the proton once.) For such large-n states, the orbital frequency is about equal to the frequency of the photon emitted in a transition from the nth level to an adjacent level with n + 1 or n- 1. Given this, find the frequency and corresponding period of the electron's orbit by computing the frequency associated with the transition from n = 100 to n = 101. Using your values for the electron's orbital size (distance) and travel time (period), calculate the approximate speed of the electron in the 100th orbit. How does this speed compare to the speed of light?
5. In the Bohr model for hydrogen, the radius of the nth orbit can be shown to be ³ times the radius of the first Bohr orbit r,-0.05 nm (see Example 10.3). Similarly, the energy of an electron in the nth orbit is times its energy when in the n 1 orbit. What is the circumference of the n = 100 orbit? (This is the distance the electron has traveled after having revolved around the proton once.) For such large-n states, the orbital frequency is about equal to the frequency of the photon emitted in a transition from the nth level to an adjacent level with n + 1 or n- 1. Given this, find the frequency and corresponding period of the electron's orbit by computing the frequency associated with the transition from n = 100 to n = 101. Using your values for the electron's orbital size (distance) and travel time (period), calculate the approximate speed of the electron in the 100th orbit. How does this speed compare to the speed of light?
Chapter10: Atomic Physics
Section: Chapter Questions
Problem 8Q
Related questions
Question
Transcribed Image Text:Home | bartleby
ong
that
n245qhp7a?filename=Bord%2C%20Donald%20J. Ostdiek%2C%20
ich
the
on
n the
g orbit
ies of
rent
ld be
434
osphere
sity of
an has
eric
eriod of
How long
al allowed
× New tab
of 562
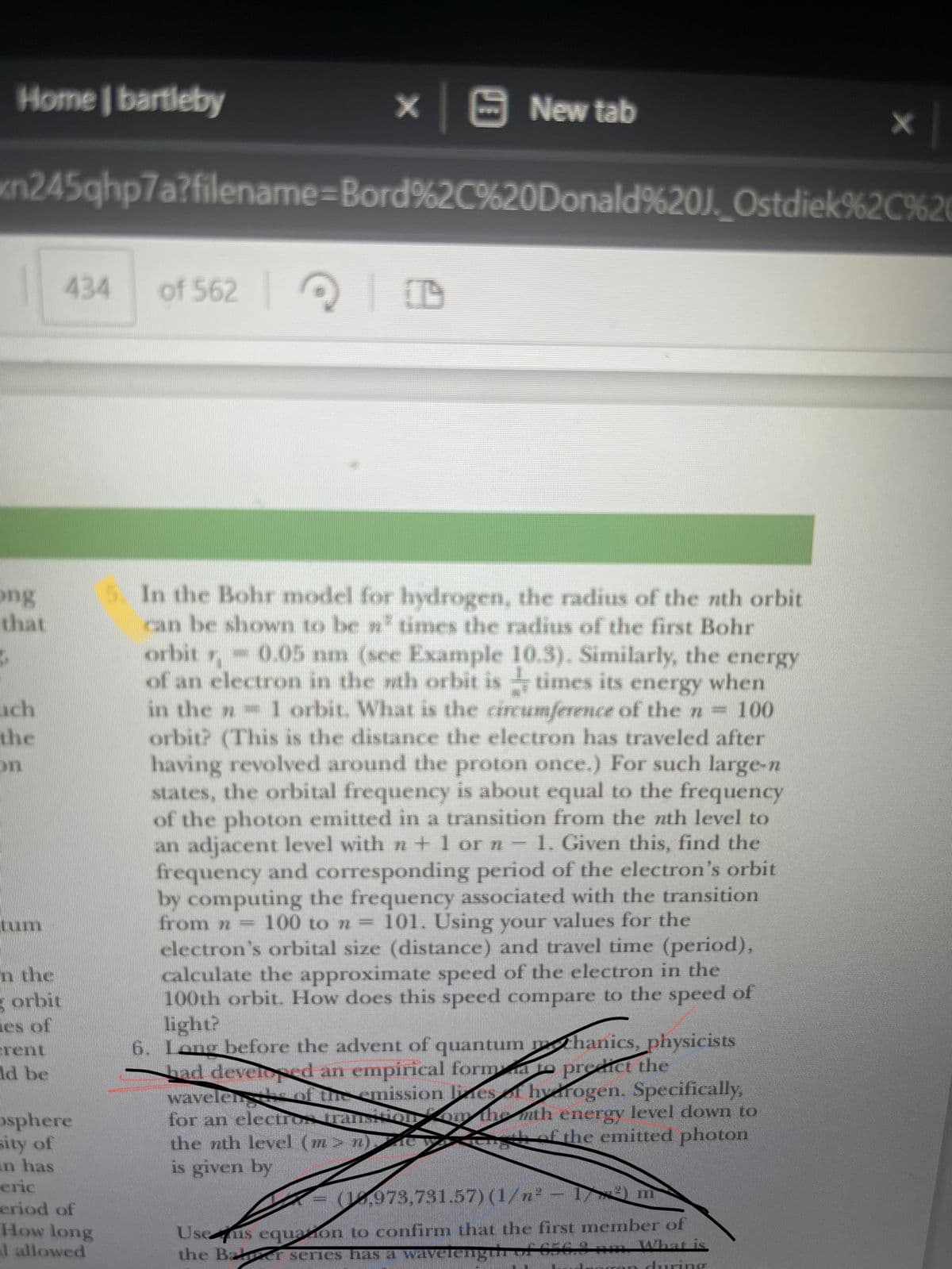
5. In the Bohr model for hydrogen, the radius of the nth orbit
can be shown to be »² times the radius of the first Bohr
orbit = 0.05 nm (see Example 10.3). Similarly, the energy
of an electron in the nth orbit is times its energy when
in the - 1 orbit. What is the circumference of the n = 100
orbit? (This is the distance the electron has traveled after
having revolved around the proton once.) For such large-n
states, the orbital frequency is about equal to the frequency
of the photon emitted in a transition from the nth level to
an adjacent level with n + 1 or n - 1. Given this, find the
frequency and corresponding period of the electron's orbit
by computing the frequency associated with the transition
100 to n = 101. Using your values for the
electron's orbital size (distance) and travel time (period),
calculate the approximate speed of the electron in the
100th orbit. How does this speed compare to the speed of
light?
from n =
6. Long before the advent of quantum mthanics, physicists
had developed an empirical formato predict the
wavelend of the emission lines
hydrogen. Specifically,
for an electros Transion on the mth energy level down to
the nth level (m> n)"
of the emitted photon
is given by
X
(1,973,731.57) (1/n² - 1²) m
Useis equazon to confirm that the first member of
the Bar series has a wavelength of 656,8 nm
What is
on during
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Horizons: Exploring the Universe (MindTap Course …
Physics
ISBN:
9781305960961
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning


Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Horizons: Exploring the Universe (MindTap Course …
Physics
ISBN:
9781305960961
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning