56Fe 1.4 4Не 235U 1.2 Fission (Splitting nuclei releases energy). 1.0 0.8 0.6 Fusion 0.4 (Combining nuclei releases energy) 0.2 20 40 60 80 100 120 160 180 200 220 240 Mass Number Binding energy per nucleon (10 12) Increased stability
56Fe 1.4 4Не 235U 1.2 Fission (Splitting nuclei releases energy). 1.0 0.8 0.6 Fusion 0.4 (Combining nuclei releases energy) 0.2 20 40 60 80 100 120 160 180 200 220 240 Mass Number Binding energy per nucleon (10 12) Increased stability
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter21: Nuclear Chemistry
Section: Chapter Questions
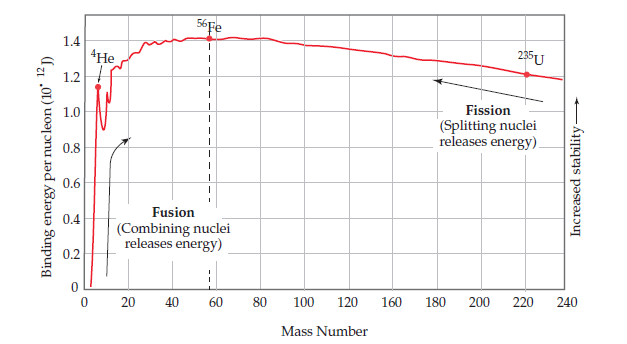
Problem 24E: Explain, in terms of Figure 21.2, how unstable heavy nuclides (atomic number > 83) may decompose to...
Related questions
Question
The
and lithium-6 are 2.014102 amu, 4.002602 amu, and
6.0151228 amu, respectively. For each isotope, calculate
(a) the nuclear mass, (b) the nuclear binding energy,
(c) the nuclear binding energy per nucleon. (d) Which of
these three isotopes has the largest nuclear binding energy
per nucleon? Does this agree with the trends plotted in
Figure ?
Transcribed Image Text:56Fe
1.4
4Не
235U
1.2
Fission
(Splitting nuclei
releases energy).
1.0
0.8
0.6
Fusion
0.4
(Combining nuclei
releases energy)
0.2
20
40
60
80
100
120
160
180
200
220
240
Mass Number
Binding energy per nucleon (10 12)
Increased stability
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning