6)- For the substitution reaction given below, determine all possible products, and indicate the favorability of each product by using numbers. For example, if product "A" is more favorable than B, and B more favorable than C, then A is labeled 1, B (2) and C (3). "Draw the structure(s) of each product and place appropriate numbers immediately next to the corresponding _structure" Answer(# of Chiral Centers): CI ONa CI CI CI Cl THF Solvent
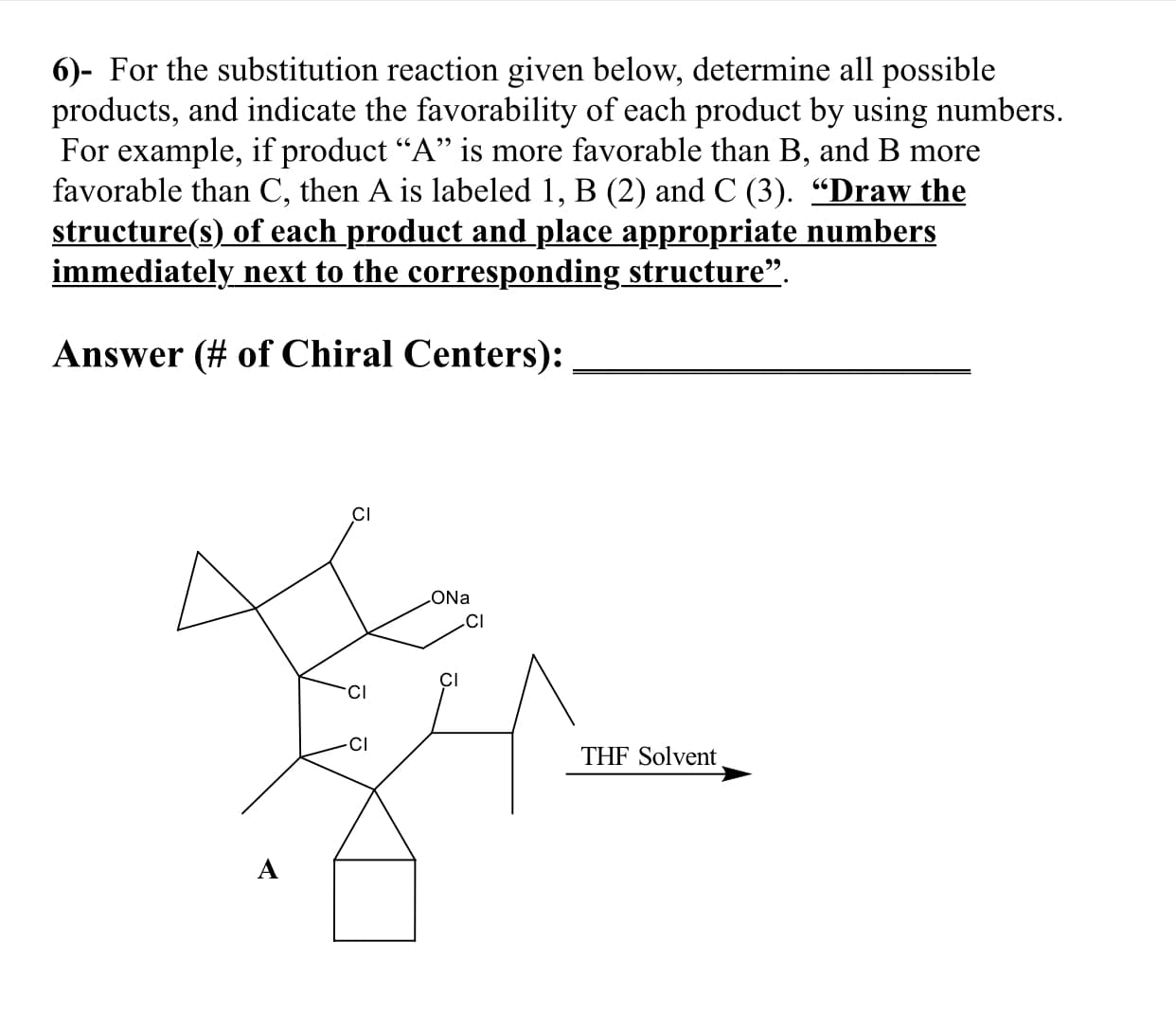
Substitution reaction:
SN1 reaction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic acid which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergoes SN1substitution reaction.
SN2 reaction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group which yield the corresponding inversion product.
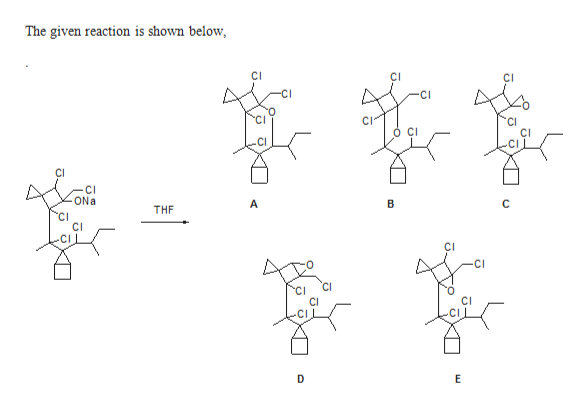
The given Compound undergoes substitution reaction gives the corresponding products like compound A, compound B, and compound C, D & E.
Compound A is more favourable than compound B, because the formation of stable six member ring through back side attack.
Compound B is more favourable than compound C, D & E, because formation of tertiary carbocation and followed by nucleophilic attack.
Compound C, D & E are three member cyclic ring , therefore ring strain is more. therefore less favoured.
Step by step
Solved in 3 steps with 3 images






