6. A student titrates 3 samples of KHP. The experimental data collected is shown below. Titration # KHP (g) | Vínal (mL) | Vmital (mL) | Votal NaOH (mL) 20.50 45.f. 1 0.7415 22.05 1.55 2 0.7115 40.80 22.05 18.75 3 0.7273 21.03 1.35 19.68 a) What is the molarity of NaOH using the data from titration 1? Show all steps in
6. A student titrates 3 samples of KHP. The experimental data collected is shown below. Titration # KHP (g) | Vínal (mL) | Vmital (mL) | Votal NaOH (mL) 20.50 45.f. 1 0.7415 22.05 1.55 2 0.7115 40.80 22.05 18.75 3 0.7273 21.03 1.35 19.68 a) What is the molarity of NaOH using the data from titration 1? Show all steps in
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.93QE
Related questions
Question
Corrects the ones that are marked wrong(6a, 6e, and 7) completely and SHOW ALL CALCULATIONS:
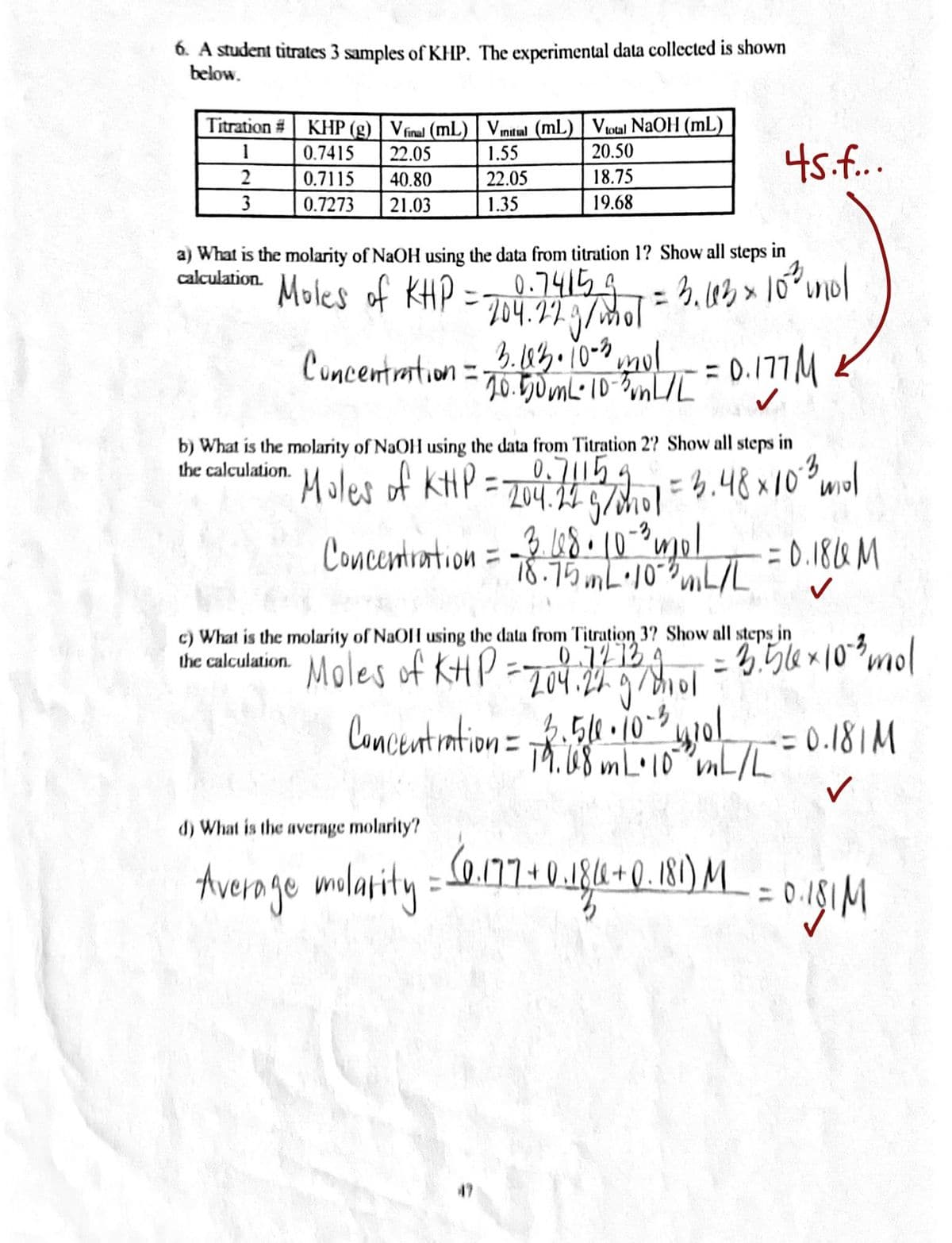
Transcribed Image Text:6. A student titrates 3 samples of KHP, The experimental data collected is shown
below.
KHP (g) | Vfinal (mL) | Vmtal (mL) | Votal NAOH (mL)
0.7415
Titration #
45.f..
1
22.05
1.55
20.50
0.7115
40.80
22.05
18.75
3
0.7273
21.03
1.35
19.68
a) What is the molarity of NaOH using the data from titration 1? Show all steps in
calculation.
Moles of KHP =.74154
204.27.9/mol
3.43.10-0ml
70.50ML-10-8nL/L
%3D
Cuncentration =
b) What is the molarity of NaOH using the data from Titration 2? Show all steps in
the calculation.
Moles of kHP =
0,71154
- 204.14 5/hol
=4.48x102mol
3. 68.10
-2 1*uol
75 mL•10-L/L
= 0.184 M
Concentration =
c) What is the molarity of NaOH using the data from Titration 3? Show all steps in
the calculation. Moles of KHP
0.7213 4
204.24
ニ
2.510.10-5,
Concentintion= Th:08 L10 vnL/L
= 0.181M
%3D
Th.08 m L•10"mL/L
d) What is the average molarity?
184+(

Transcribed Image Text:What is the relative standard deviation of the molarities? Show all steps in the
caleulation. Standurd Deviation
= S = 2(Xi-X)-
%3D
S= 0.177-0.181) M+ (0-184 - 0.181)-M +(0.181-0.181 M
= 4.528 ×10- M
X 1000
Relative Standard eviation = 4.528×10°M
100
D.181 M
0.004528M.
0.181 M
X 100 =2-500%0
Y Is the data within the precision standard?,
No, the data is nit within, the precision standard since
the fint valve, 0.177 is not, close' to the seçond valve,
0.184. Even though the relative standard devintion is
ouly 2.50%,the 4truth is that it can be improved.
8. Should the student perform more titrations?
order to reduce even 'more +hote tritrations
And thercfore, the relative rtandard deviation
of the wmorality of NaCH.
Yes the student sliould perfurm, more
or der to reduce even 'more the standard deviation
48
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
