6. Analysis of Buffers in Unknown Imagine that you are given thre is a buffer, the other two are no pipettes, pH paper, and a soluti would you determine which so answer this question.
6. Analysis of Buffers in Unknown Imagine that you are given thre is a buffer, the other two are no pipettes, pH paper, and a soluti would you determine which so answer this question.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 75P
Related questions
Question
answer question 6
if needed use the in the picture
Transcribed Image Text:ugar
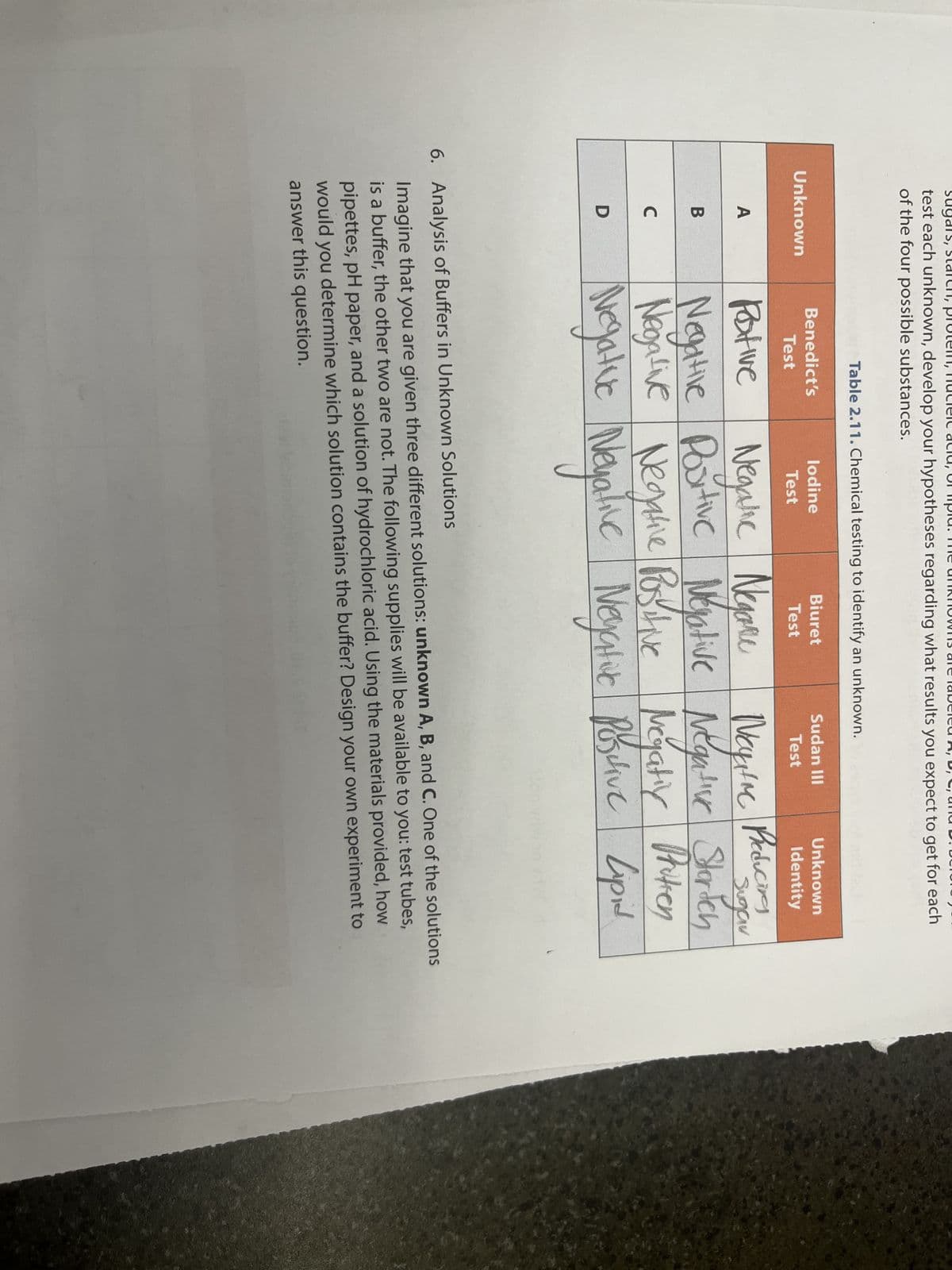
test each unknown, develop your hypotheses regarding what results you expect to get for each
of the four possible substances.
Unknown
A
B
C
D
Table 2.11. Chemical testing to identify an unknown.
Benedict's
Test
lodine
Test
Biuret
Test
Sudan III
Test
Unknown
Identity
Preducing
Pative
Negalic Negaru
Neyime
Storden
Negative Portive Negative Negative Storich
Negative Negative Positive Negativ Protien
Lipid
Negative Negative Negatic Posclive Lapal
6. Analysis of Buffers in Unknown Solutions
Imagine that you are given three different solutions: unknown A, B, and C. One of the solutions
is a buffer, the other two are not. The following supplies will be available to you: test tubes,
pipettes, pH paper, and a solution of hydrochloric acid. Using the materials provided, how
would you determine which solution contains the buffer? Design your own experiment to
answer this question.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning