Q: What is the conjugate acid of HBO32-
A: Given base, HBO32- this conjugate base formed in the second ionization of boric acid.
Q: Identify the acid and conjugate base in each reaction. Calculate the pKA for each acid. List them in…
A: Conjugate acid-base pair are considered differ only by H+ ion. Acid form corresponding conjugate…
Q: What is the pKb of the conjugate base of a weak acid that has a pKa equal to 3.52? (temperature is…
A: Given that : pKa = 3.52 pKb = ?
Q: Write the equilibrium-constant expressions and obtain numerical values for each constant in (a) the…
A: Acid dissociation constant Ka is utilized to determine the acidic and basic nature of a solution. It…
Q: How does the pKe of the conjugate acid of benzylamine (CgH5CH,NH2) compare to the pk,'s of the…
A: pKa is inversely proportional to acidity strength. A more acidic molecule will have lower pKa value.…
Q: What is the pKb of the conjugate base of a weak acid that has a pKa equal to 5.29 at 25ºC?
A: To get the pKb of the base (B) you MUST subtract the pKa from 14. The reason for this is that the…
Q: A Keq value of 5.2 x 104 tells us that, at equilibrium, ____________ are favored. Group of answer…
A: The equilibrium constant of a reaction in given by: Keq = [Products][Reactants] If the…
Q: 4. Dr Atiqah analysing the structure of one common type of biomolecule and found that it has a…
A: Crosslinking in proteins implies the process of chemically attaching two or more protein chains by a…
Q: Treatment of indene with NaNH2 forms its conjugate base in a Brønsted–Lowry acid–base reaction. Draw…
A:
Q: uestion 67 Drag the appropriate components onto the blank expression to give the correct acid…
A:
Q: Which of the following has the lowest pKa value? Hint: a methyl group is an electron donating group…
A:
Q: a) The weaker acid is b) Its conjugate base is c) The species that predominate at equilibrium are…
A: SOLUTION: Step 1: According to the Bronsted-Lowry theory of acids and bases, an acid is a molecule…
Q: 6. For each of the following reactions schemes: a. Draw in the mechanistic curved arrows (each of…
A:
Q: For the following reaction: он `NH a-Label the acid and base in the starting materials. b-Draw the…
A: The given reaction is : CH3CH2CH2NH- + C5H10O ------------> CH3CH2CH2NH2 + C5H9O- Acid :- Is the…
Q: The pKa values of the carboxylic acid groups of oxaloacetic acid are 2.22 and 3.98.a. Which carboxyl…
A: a. The structure of oxaloacetic acid is; The pKa value is inversely related to the acidic strength…
Q: Write the equilibrium-constant expressions and obtain numerical values for each constant in (a) the…
A: Hello. Since your question has multiple sub-parts, we will solve the first three sub-parts for you.…
Q: 2. What are the pKa's of HCN (hydrocyanic acid), and HF (hydrofluoric acid)? Based only on those pka…
A: We have to write the pka's of HCN (hydrocyanic acid), and HF (hydrofluoric acid). Based only on…
Q: 1. Write the equilibrium-constant expressions and obtain numerical values for each constant in (a)…
A: We'll answer the first question with three sub parts, since the exact one wasn't specified. Please…
Q: 5. Which one of these molecules would you expect to be the stronger base? Justify your answer. N.
A: For the given nitrogen containing compounds in the question, localization of the lone pair of…
Q: c) Draw the product of the following Lewis acid-base reaction. Discuss whether the product will…
A: AlCl3 is an Lewis acid which is able to accept lone pairs of any atom in its vacant d Orbital.
Q: Which of the following has the lowest pKa value? Hint: a methyl group is an electron donating group…
A:
Q: Using pKa Values to Determine Relative Acidity and Basicity Rank the following compounds in order of…
A: pKa of acid : Ka is called the acid dissociation constant. If any compound easily gives H+ than the…
Q: 3. Calculate the pKa value of hydroxylammonium cation (HO-H3N*)(protonated on nitrogen hydroxyl…
A:
Q: Treatment of indene with NaNH2 forms its conjugate base in a Brønsted– Lowry acid–base reaction.…
A:
Q: Consider the following compounds that vary from nearly nonacidic to strongly acidic. Draw the…
A:
Q: (3 pts) For the following sets of compounds, place the compounds in order of increasing acidity, and…
A: Acidity is measured as strength to loose a H atom or H+ ion. (a) The increasing order of acid…
Q: (a)Which side for the each of the following acid-base equilibria would be primarily favored?…
A: Here we have to predict the acid-base reactions which are primarily equilibrium favoured and…
Q: CH;COOH +NH; CH;COO+NH, 9. In the above reaction in aqueous solution, the acid reactant is and its…
A: According to Lewis theory acid is a substance that accepts electrons and base is a substance that…
Q: Which species is the stronger Lewis Acid? Why? (Use resonance structures to explain your answer.)…
A: Lewis acid are electron deficient species , that have ability to accept electron from Lewis base…
Q: What is the pKg of its conjugate base?
A: Given :- Ka = 7.89 × 10-8 Calculate :- pKb of the conjugate base
Q: The pKa of acetic acid in water is 4.76. What effect will a decrease in the polarity of the solvent…
A: Acetic acid in water has a pKa value of 4.76.
Q: CH CN OCH, 3. .32 312 Ce 231 4. 3.2 1 123 1 213
A: Acidity is defined as the ability to lose the hydrogen ion. The molecule that loses the proton more…
Q: I don't understand the pKa equation. What is the "p"? What does negative logarithm mean?
A:
Q: Thiocyanic acid (HSCN) is an inorganic acid (pKa = 1.1 at 25 °C) that can be classified as “strong”…
A:
Q: Aniline (conjugate acid pKa 4.63) is a considerably stronger base than diphenylamine (pKa 0.79).…
A: In aniline the lone pair of nitrogen atom is delocalized on one benzene ring only.
Q: Acetic acid (pKa = 4.75) is contained in vinegar. What is the pKb of the conjugate base of acetic…
A:
Q: Phenoxide ion (C,H5O") is a weak base, with K, = 7.70 x 105. What is the pKa of the phenol, C,H5OH…
A: Solution : Bronsted-Lowry distinguished acid and base on the basis of their proton donating or…
Q: Which of the following statements about the dissociation constant and pka is NOT correct?
A: An acid dissociation constant (Ka) is defined as a quantitative measure of an acid strength in…
Q: 6. Rank the following protons in order of decreasing acidity (HA - HD). Explain your answer using…
A: The molecule given is,
Q: Which of the following bases has a conjugate acid with the smallest pKa? Select one: O (CH,CH,CH,),N…
A:
Q: For an acid HA with a pKa of 5.2, what is the ratio of the concentration of the conjugate base to…
A: The dissociation reaction of acid HA can be written as => HA ------> H+ + A- Conjugate base…
Q: The hydrolysis of the ester shown here is catalyzed by morpholine. Explain how morpholine catalyzes…
A:
Q: If the pKa of a weak acid HA is 3.84, what is the pkb of its conjugate base A-
A: Given Data : pKa = 3.84 To Find : pKb
Q: The pKa values of the carboxylic acid groups of oxaloacetic acid are 2.22 and 3.98. a. Which…
A: a. The structure of oxaloacetic acid is; The pKa value is inversely related to the acidic strength…
Q: What is the Ka expression for this reaction? HC2H3O2(aq) + H2O(1) H30*(aq) + C2H3O2°(aq)
A: The equilibrium constant for the given expression is known as the acid dissociation constant (Ka).
Q: For an acid with a pKa of 5.00, what is the pKb of its conjugate base?
A:
Q: . For the following acid-base reaction, i) complete the acid-base reaction by writing the products…
A:
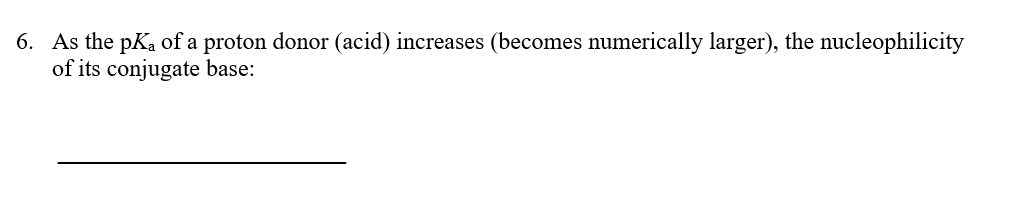
1) High pKa value means weak acid,
low pKa value means strong acid.
2) Strong acid have weak conjugate base
and Weak acid have strong conjugate base.
And a conjugate base is always a stronger nucleophile.
Step by step
Solved in 2 steps

- What is the conjugate acid of acetamide and its pka value?Acetic acid can also react as a very weak base (pKb = 20). Two different sites on acetic acid might become protonated to give the conjugate acid. Draw both of these possible conjugate acids, and explain (resonance) why the correct one is more stable. Calculate the pKa of this conjugate acid.Treatment of indene with NaNH2 forms its conjugate base in a Brønsted– Lowry acid–base reaction. Draw all reasonable resonance structures for indene's conjugate base, and explain why the pKa of indene is lower than the pKa of most hydrocarbons.
- Treatment of indene with NaNH2 forms its conjugate base in a Brønsted–Lowry acid–base reaction. Draw all reasonable resonance structures for indene’s conjugate base, and explain why the pKa of indene is lower than the pKa of most hydrocarbons.Calculate the pKa of a acid at 25°C if its conjugate base has a pKb = 1.19Arrange the compounds in each set in order of increasing base strength. consult Table 4.1 for pKa values of the conjugate acid of each base.
- explain in great detail why the pKa values are what they are and compare them among the three compounds. The conjugate acids that are protonated are drawn in red for each compound. Which one based on the pKa is most acidic and what does that tell you about how basic their lone pairs are?What percent of acetic acid is present in the acidic form at pH 5.0, assuming a pKa of 4.8?The pKa of our product is ~ 2-3 units lower than the pKa of phenol. Draw all possible resonance structures of the conjugate base of the product, and use these to explain why our product is so much more acidic than phenol.
- Which acid do you think would have the lower pKa value: HBrO4, HBrO3, HBrO2, or HBrO? Explain your answer.Verify that the equilibrium position for the reaction between phenol and hydroxide ion is on the right by comparing the pKa value of the acid on the left with that of the acid on the right. Which acid is stronger? do the same for the reaction of phenol with hydrogen carbonate ionWhich is the conjugate base in each of the pairs below?

