6. Based on a series of experiments, a third year chemistry student observes the following interactions between solid metals and their ions (Table 1). able 1: Reactions between selected metals and their ions. (modified from van Kessel et al., 2003) Metal / lon A1*+, (aq) TI* (aq) Ga3+ In3+ (aq) (aq) spontaneous spontaneous spontaneous no reaction no reaction no reaction Gag) no reaction spontaneous spontaneous Ing no reaction spontaneous no reaction repare a redox table of half-reactions showing the relative strengths of oxidizing and reducing agents ased on these observations. Explain your choices using point form notes to facilitate your reader's nderstanding.
6. Based on a series of experiments, a third year chemistry student observes the following interactions between solid metals and their ions (Table 1). able 1: Reactions between selected metals and their ions. (modified from van Kessel et al., 2003) Metal / lon A1*+, (aq) TI* (aq) Ga3+ In3+ (aq) (aq) spontaneous spontaneous spontaneous no reaction no reaction no reaction Gag) no reaction spontaneous spontaneous Ing no reaction spontaneous no reaction repare a redox table of half-reactions showing the relative strengths of oxidizing and reducing agents ased on these observations. Explain your choices using point form notes to facilitate your reader's nderstanding.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 6CR: How is the pH scale defined? What range of pH values corresponds to acidic solutions? What range...
Related questions
Concept explainers
Question
Pls help ASAP
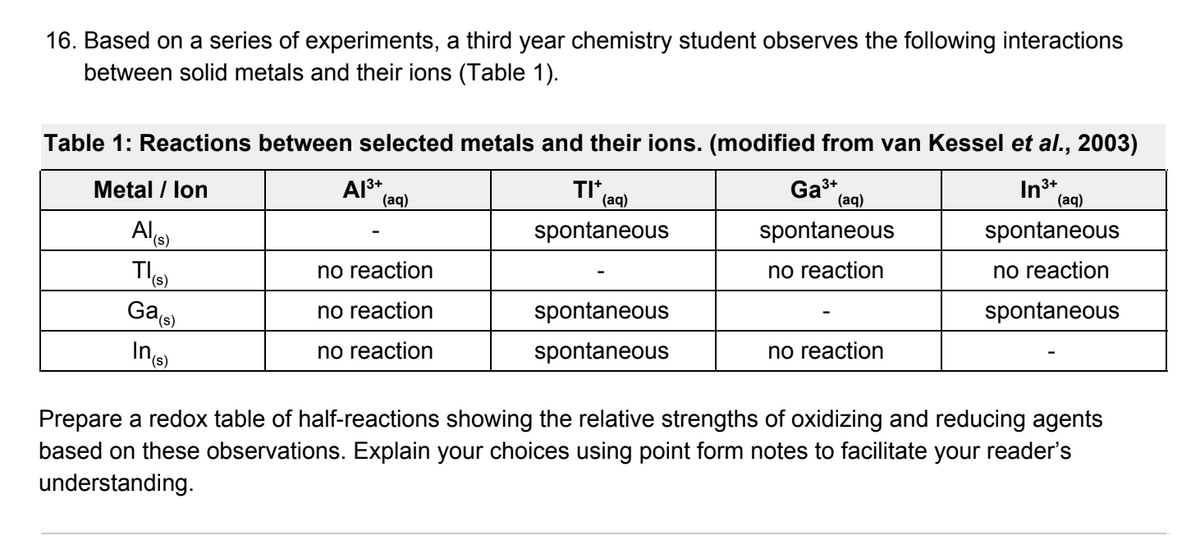
Transcribed Image Text:16. Based on a series of experiments, a third year chemistry student observes the following interactions
between solid metals and their ions (Table 1).
Table 1: Reactions between selected metals and their ions. (modified from van Kessel et al., 2003)
Metal / lon
TI*
(aq)
Ga3+
In3+
(aq)
(aq)
(aq)
Al
spontaneous
spontaneous
spontaneous
no reaction
no reaction
no reaction
Gae
no reaction
spontaneous
spontaneous
no reaction
spontaneous
no reaction
Prepare a redox table of half-reactions showing the relative strengths of oxidizing and reducing agents
based on these observations. Explain your choices using point form notes to facilitate your reader's
understanding.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning