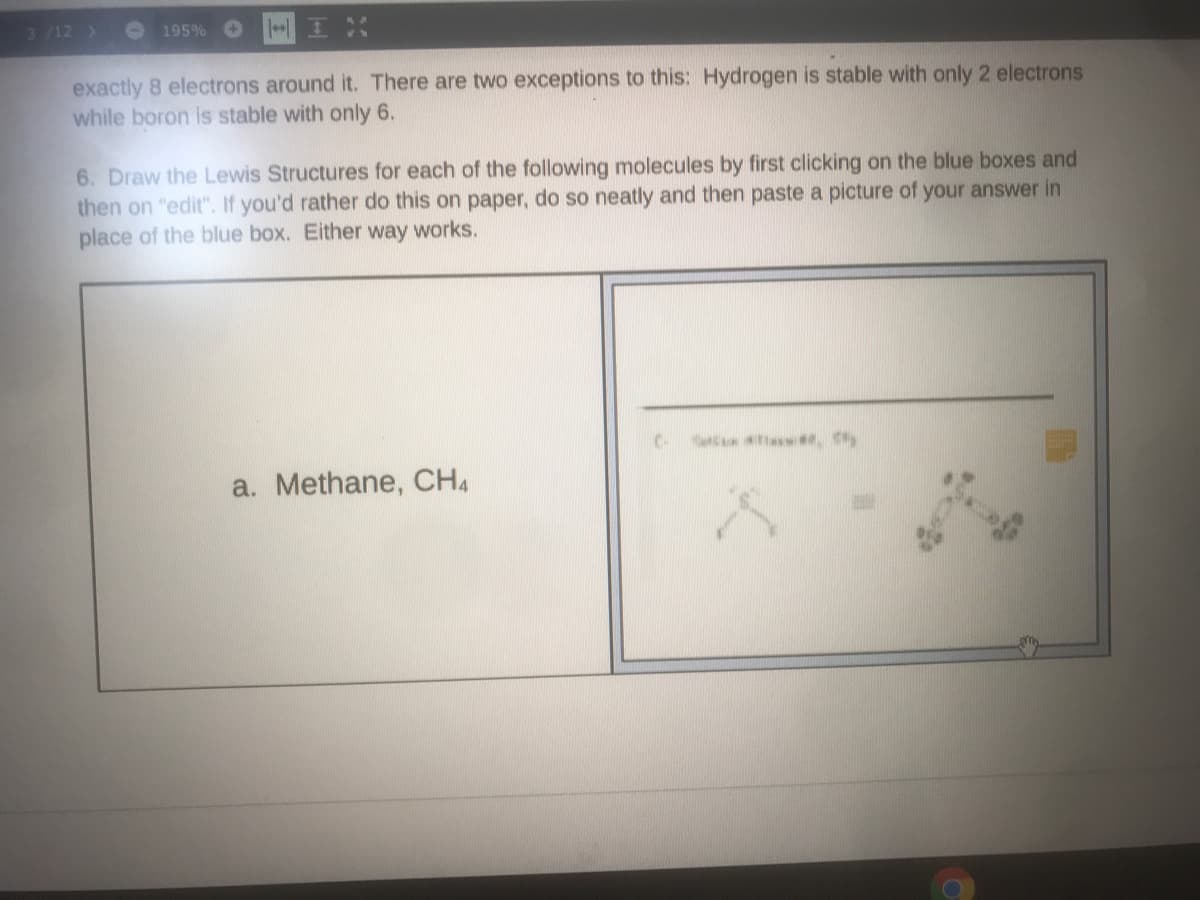
6. Draw the Lewis Structures for each of the following molecules by first clicking on the blue boxes and then on "edit". If you'd rather do this on paper, do so neatly and then paste a picture of your answer in place of the blue box. Either way works. CR we, y a. Methane, CH4
6. Draw the Lewis Structures for each of the following molecules by first clicking on the blue boxes and then on "edit". If you'd rather do this on paper, do so neatly and then paste a picture of your answer in place of the blue box. Either way works. CR we, y a. Methane, CH4
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 2CTQ: The valence shell of an atom in a legitimate Lewis structure (see Figure 2.3) has what in commonwith...
Related questions
Question
You did this question for me but my teacher said its wrong can you please fix it
Transcribed Image Text:3 /12 >
195%
exactly 8 electrons around it. There are two exceptions to this: Hydrogen is stable with only 2 electrons
while boron is stable with only 6.
6. Draw the Lewis Structures for each of the following molecules by first clicking on the blue boxes and
then on "edit". If you'd rather do this on paper, do so neatly and then paste a picture of your answer in
place of the blue box. Either way works.
C.
C w , Sty
a. Methane, CH4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning