6. From the following enthalpies of reaction find AHan for 2 HCl(g) + F₂(g) ---> 2 HF(1) + Cl₂2(g) AHrxn= ? 4 HCl(g) + O₂(g) ---> 2 H₂O() +2 Cl₂(g) 1/2 H₂(g) + 1/2 F₂(g) ---> HF(1) H₂(g) + 1/2 O₂(g) ---> H₂O(l) a) +766 kJ/mol b) -988.4 kJ/mol AH = -148.4 kJ/mol AH-600.0 kJ/mol AH = -285.8 kJ/mol Ⓒ-840 kJ/mol d) +1337 kJ/mol
6. From the following enthalpies of reaction find AHan for 2 HCl(g) + F₂(g) ---> 2 HF(1) + Cl₂2(g) AHrxn= ? 4 HCl(g) + O₂(g) ---> 2 H₂O() +2 Cl₂(g) 1/2 H₂(g) + 1/2 F₂(g) ---> HF(1) H₂(g) + 1/2 O₂(g) ---> H₂O(l) a) +766 kJ/mol b) -988.4 kJ/mol AH = -148.4 kJ/mol AH-600.0 kJ/mol AH = -285.8 kJ/mol Ⓒ-840 kJ/mol d) +1337 kJ/mol
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 76E: Combustion reactions involve reacting a substance with oxygen. When compounds containing carbon and...
Related questions
Question
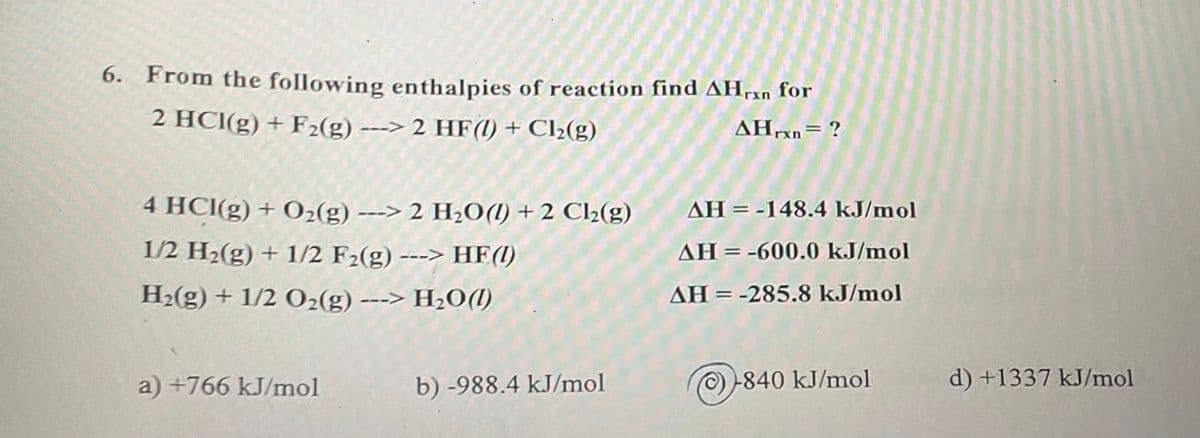
Transcribed Image Text:6. From the following enthalpies of reaction find AHan for
2 HCl(g) + F₂(g) ---> 2 HF(1) + Cl₂2(g)
AHrxn= ?
4 HCl(g) + O₂(g) ---> 2 H₂O() +2 Cl₂(g)
1/2 H₂(g) + 1/2 F₂(g) ---> HF(1)
H₂(g) + 1/2 O₂(g) ---> H₂O(l)
a) +766 kJ/mol
b) -988.4 kJ/mol
AH = -148.4 kJ/mol
AH-600.0 kJ/mol
AH = -285.8 kJ/mol
Ⓒ-840 kJ/mol
d) +1337 kJ/mol
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning