6. One electron is trapped in a one-dimensional square well potential with infinitely high sides. Assume that the well extends from x = 0 to x =L. The width of the well is L=306.6pm. Find the energies (E,,E2,E‚,E,) of the four lowest levels in electron volts (eV). a. b. Sketch the probability density functions for the two lowest energy states: P(x), P,(x). Draw an energy level diagram for the for the first four energy levels. С.
6. One electron is trapped in a one-dimensional square well potential with infinitely high sides. Assume that the well extends from x = 0 to x =L. The width of the well is L=306.6pm. Find the energies (E,,E2,E‚,E,) of the four lowest levels in electron volts (eV). a. b. Sketch the probability density functions for the two lowest energy states: P(x), P,(x). Draw an energy level diagram for the for the first four energy levels. С.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 7P
Related questions
Question
Please explain parts c, d, and e. Please include significant figures and units. Thanks for your help!
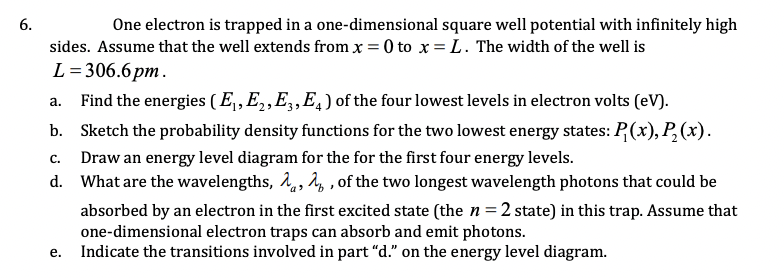
Transcribed Image Text:6.
One electron is trapped in a one-dimensional square well potential with infinitely high
sides. Assume that the well extends from x = 0 to x = L. The width of the well is
L= 306.6pm.
Find the energies (E,,E,,E,,E,) of the four lowest levels in electron volts (eV).
а.
b. Sketch the probability density functions for the two lowest energy states: P(x), P,(x).
Draw an energy level diagram for the for the first four energy levels.
C.
d. What are the wavelengths, å,, 4, , of the two longest wavelength photons that could be
absorbed by an electron in the first excited state (the n=2 state) in this trap. Assume that
one-dimensional electron traps can absorb and emit photons.
Indicate the transitions involved in part "d." on the energy level diagram.
е.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,