6. Using graph C: a. Explain what happens when hypothermia sets in (when enzymes get too cold!) Reaction Rate vs. Substrate Concentration D I Substrate Concentration b. Does the same thing happen when enzymes get too hot? Why or why not? Rate of Reaction-
6. Using graph C: a. Explain what happens when hypothermia sets in (when enzymes get too cold!) Reaction Rate vs. Substrate Concentration D I Substrate Concentration b. Does the same thing happen when enzymes get too hot? Why or why not? Rate of Reaction-
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
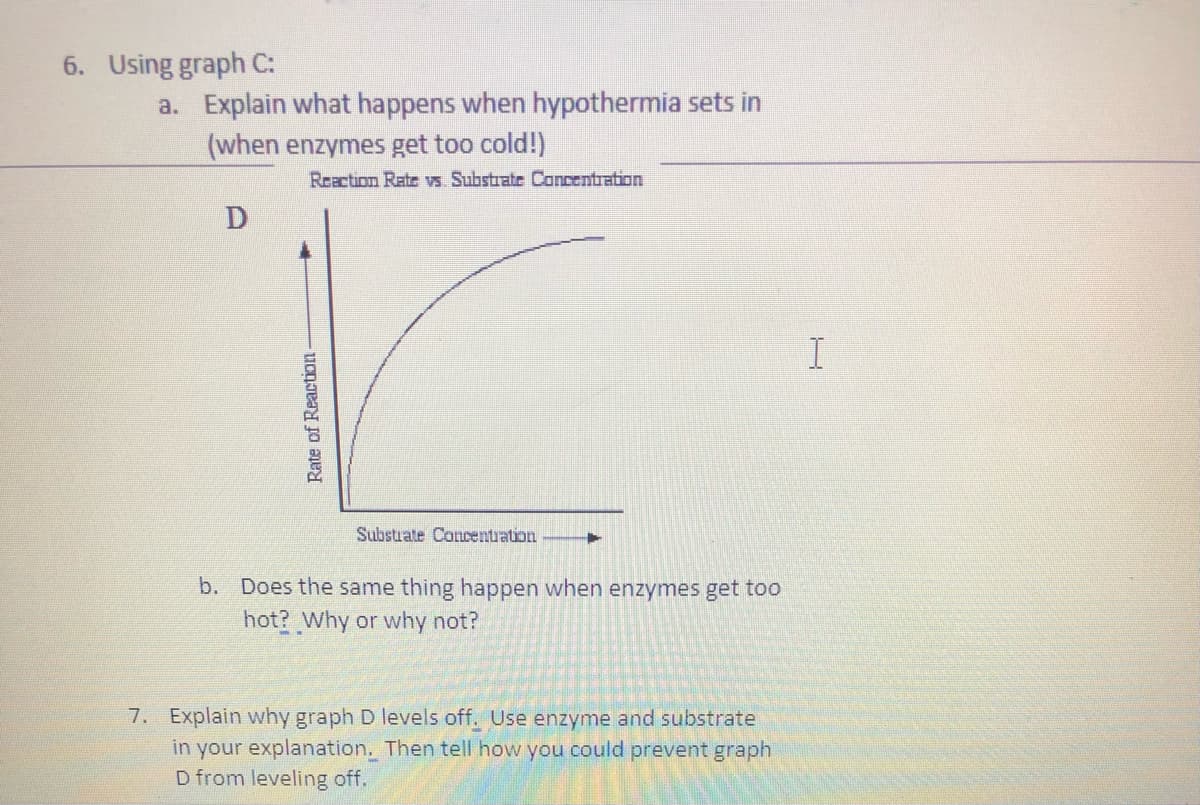
Transcribed Image Text:6. Using graph C:
a. Explain what happens when hypothermia sets in
(when enzymes get too cold!)
Reaction Rate vs Substrate Concentration
I
Substrate Concentuation
b. Does the same thing happen when enzymes get too
hot? Why or why not?
7. Explain why graph D levels off. Use enzyme and substrate
in your explanation. Then tell how you could prevent graph
D from leveling off.
Rate of Reaction -
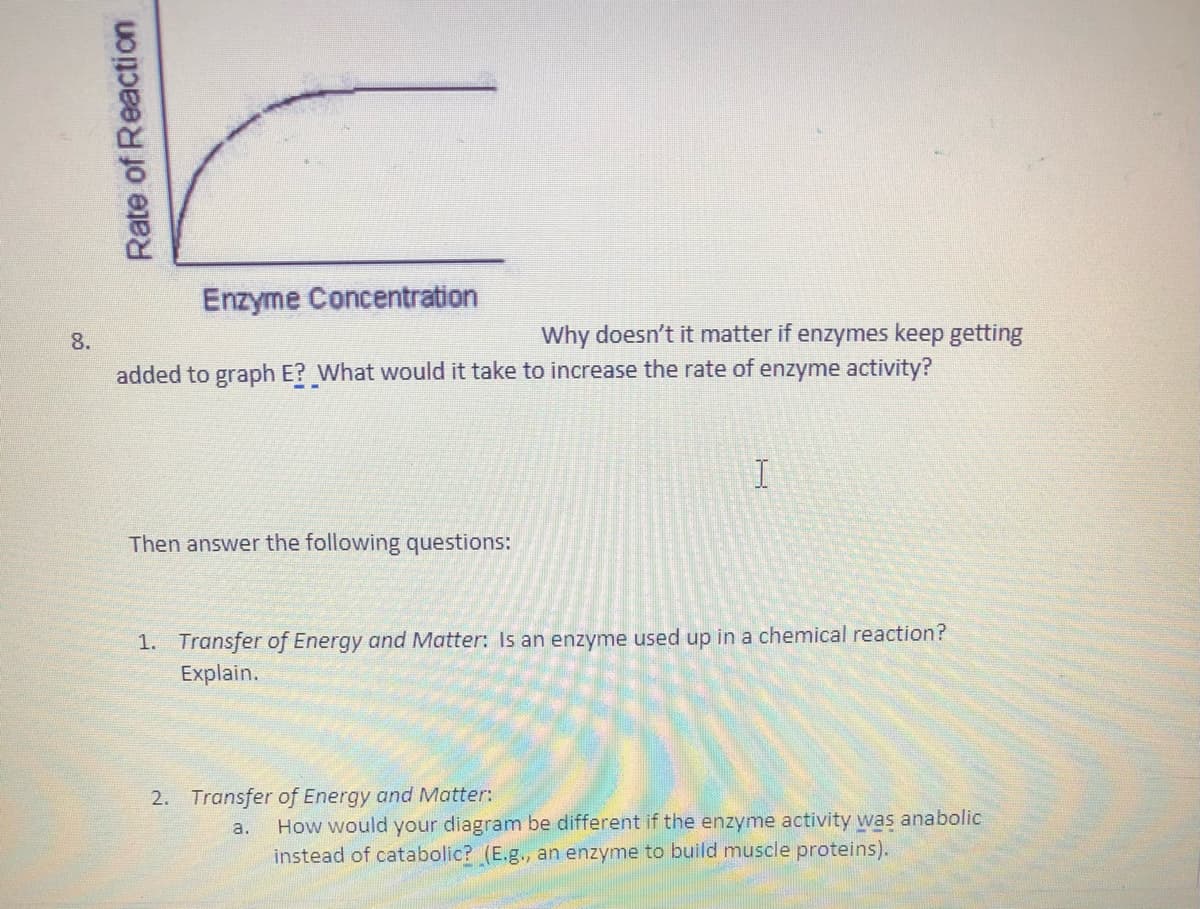
Transcribed Image Text:Enzyme Concentration
8.
Why doesn't it matter if enzymes keep getting
added to graph E? What would it take to increase the rate of enzyme activity?
Then answer the following questions:
1. Transfer of Energy and Matter: Is an enzyme used up in a chemical reaction?
Explain.
2. Transfer of Energy and Matter:
How would your diagram be different if the enzyme activity was anabolic
instead of catabolic? (E.g., an enzyme to build muscle proteins).
a.
Rate of Reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON