60. . The greater the emf of a cell (anode emf minus cathode emf), the greater the tendency that corrosion will occur. For the following corrosion cells, write the anode and cathode reactions in salt water and the initial cell emf, please see attached table. MF table ? a) Mild steel and zinc. b) 1100 aluminum and copper EMF table TABLE 20-1 The electromotive force (emf) series for selected elemerts Electrode Potential Metal (V) - 3.05 - 2.37 - 1.66 - 1.63 - 1.63 -0.76 - 0.74 - 0.44 Anodic Li - Li* + c* Mg - Mg* + 2e Al + Al+ + 3e Ti* + 2e" Mn2+ + 2e" Ti - Mn Zn -- Zn* + 2e Čř + Cr* + 3e" Fe . Fe+ + 2c- Ni Ni+ + 2c' Sn -+ Sn* + 2e Pb - Pb?* + 2c" -0.25 - 0.14 -- 0.13 0.00 +0.34 +0.40 + 0.80 + 1.20 + 1.23 + 1.5 H2 + 2H* + 2e" Cu - Cu?+ + 2c" 4(OH)" + O2 + 2H,O + 4e- Ag - Ag* + e- Pt Pt* + 4e¯ 2H,0 + 0, + 4H + 4c- Au+ + 3c" Cathodic Au -
60. . The greater the emf of a cell (anode emf minus cathode emf), the greater the tendency that corrosion will occur. For the following corrosion cells, write the anode and cathode reactions in salt water and the initial cell emf, please see attached table. MF table ? a) Mild steel and zinc. b) 1100 aluminum and copper EMF table TABLE 20-1 The electromotive force (emf) series for selected elemerts Electrode Potential Metal (V) - 3.05 - 2.37 - 1.66 - 1.63 - 1.63 -0.76 - 0.74 - 0.44 Anodic Li - Li* + c* Mg - Mg* + 2e Al + Al+ + 3e Ti* + 2e" Mn2+ + 2e" Ti - Mn Zn -- Zn* + 2e Čř + Cr* + 3e" Fe . Fe+ + 2c- Ni Ni+ + 2c' Sn -+ Sn* + 2e Pb - Pb?* + 2c" -0.25 - 0.14 -- 0.13 0.00 +0.34 +0.40 + 0.80 + 1.20 + 1.23 + 1.5 H2 + 2H* + 2e" Cu - Cu?+ + 2c" 4(OH)" + O2 + 2H,O + 4e- Ag - Ag* + e- Pt Pt* + 4e¯ 2H,0 + 0, + 4H + 4c- Au+ + 3c" Cathodic Au -
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.16QAP
Related questions
Question
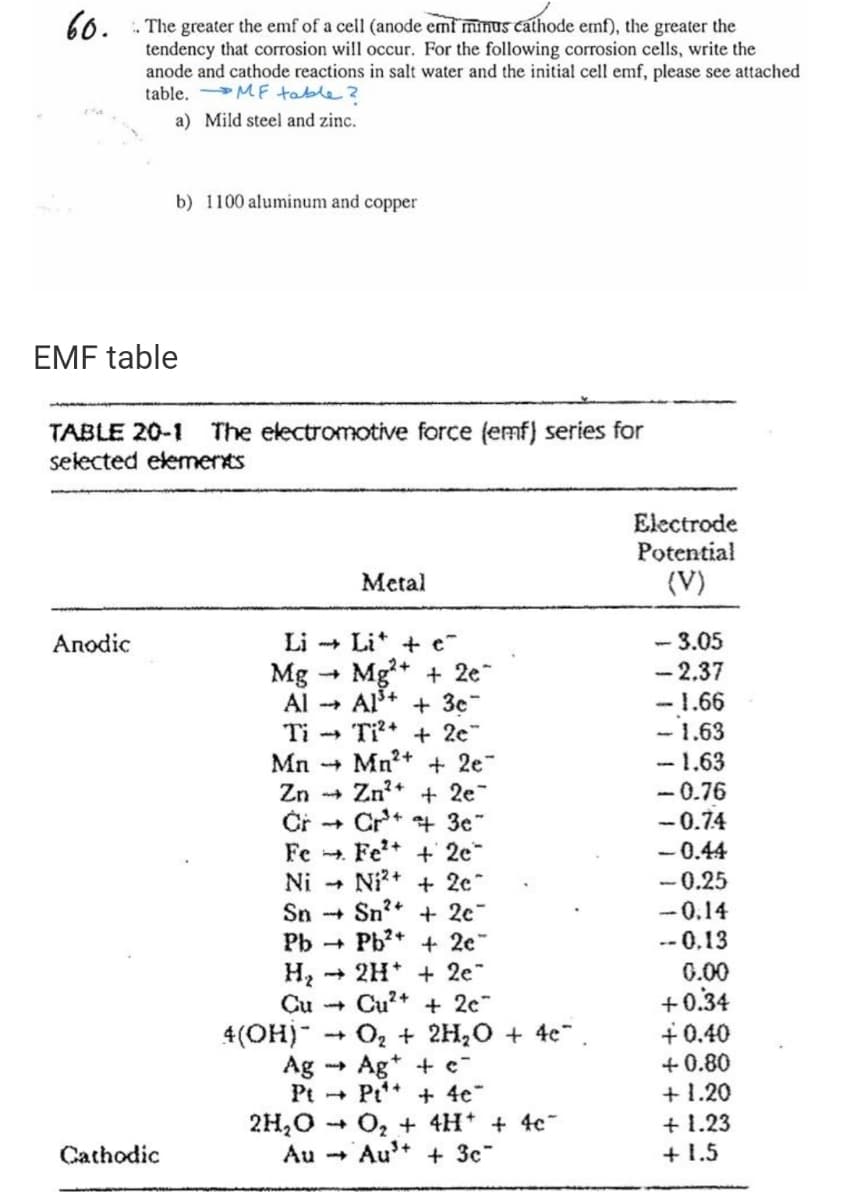
Transcribed Image Text:60.
. The greater the emf of a cell (anode emf minus cathode emf), the greater the
tendency that corrosion will occur. For the following corrosion cels, write the
anode and cathode reactions in salt water and the initial cell emf, please see attached
table. MF table ?
a) Mild steel and zinc.
b) 1100 aluminum and copper
EMF table
The electromotive force (emf) series for
TABLE 20-1
selected elemerts
Electrode
Potential
Metal
(V)
Li + Lit + e
Mg* + 2e
Al+ + 3c"
Ti* + 2e"
Mn2+ + 2e"
Zn* + 2e
Čř + Cr+
Fe .
- 3.05
- 2.37
-1.66
- 1.63
- 1.63
-0.76
Аnodic
Mg
Al --+
Ti
Mn +
Zn -
- 0.74
- 0.44
- 0.25
- 0.14
-- 0,13
+ 3e"
Fe* + 2e
Ni2+ + 2c"
Sn -+ Sn?* + 2e
Pb + Pb?* + 2c"
Ni
+ 2H* + 2e"
0.00
+0.34
+ 0.40
+ 0.80
+ 1.20
+ 1.23
+ 1.5
Cu -
Cu²+ + 2c"
Oz + 2H,0 + 4e".
Ag
Pt Pt* + 4e"
2H,0
Au - Au+ + 3c"
4(OH)"
Ag* + c-
O, + 4H* + 4c
Cathodic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
